Raman spectroscopic probe aims to streamline prostate cancer therapy
Unexpected hurdles often arise when translating novel diagnostics to clinical applications. A recent study from a Canadian-led research team aims to minimize those hurdles by considering clinical workflow early in the research. In this case, the clinical problem is the identification and treatment of prostate cancer, and the promising diagnostic method is Raman spectroscopy.
Prostate cancer is the most diagnosed form of cancer in North American men, and the second-leading cause of cancer deaths in that demographic. Currently, if initial tests, such as for elevated levels of prostate-specific antigen, are abnormal, patients may be referred for an ultrasound exam. That could be combined with magnetic-resonance imaging (MRI) to create a 3D model of the prostate, which helps direct a tissue biopsy. Biopsy results then inform the direction of therapy, which may include radiation delivery. Professor Samuel Kadoury from Polytechnique Montreal and the Centre Hospitalier de l’Universite de Montreal (CHUM) leads a team working on a technique to replace several of those steps.
Locating tumor tissue
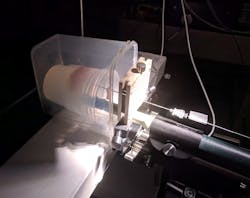
Noting that Raman spectroscopy can provide diagnostic information relating to DNA, proteins, and lipidic content, Kadoury’s team is developing a Raman optical probe that can be integrated into the standard prostate-cancer treatment flow (see figure). The probe houses eleven 100 µm optical fibers and a six-degree-of-freedom electromagnetic (EM) tracker. One of the optical fibers, the illumination fiber, is capped with a bandpass filter centered at 709 nm, allowing excitation at both 671 and 785 nm. Nine of the fibers are used for signal detection. They terminate in a 785 nm notch filter to reduce background signal from the optical fibers. The remaining detection fiber has no filter, being reserved for future investigations of the possible diagnostic value of diffuse-reflectance measurements. The fibers and EM probe are integrated in a 1.22-mm-diameter housing that widens to 2.1 mm for easier handheld manipulation.
The probe is designed to be oriented face-on, butted against the tissue to be evaluated. The signal from all nine detection fibers is routed to a single detector. Simultaneously, the EM location sensor provides orientation and position information, which is merged with MRI and/or ultrasound images to precisely identify the probe placement.
“In prostate cancer,” Kadoury says, “there have been several demonstrations of Raman spectroscopy on ex vivo samples, following a biopsy or prostatectomy, in order to confirm margins or presence of tumor with the tumor exposed tissue. However, this is the first in situ demonstration, combining the probe with an electromagnetic navigation system to confirm the location of the Raman measurements with respect to the diagnostic magnetic resonance image.” The location information is key to incorporating Raman information into the current workflow.
Of course, adding Raman spectra to diagnostic and therapeutic procedures is only of value if the spectra provide clinically relevant information, so classification is another key step in the development. Classification describes the system’s ability to distinguish, in this case, cancerous from normal tissue. Kadoury’s team analyzed 434 spectra acquired on fresh slices from 32 excised prostates. Relatively long exposure times of 60 to 300 s provided high-quality data for the classification algorithm. A pathologist then performed standard hematoxylin and eosin (H&E) staining on the samples and separated them into cancerous and healthy groups. A support-vector machine algorithm identified 39 spectral features that differed in the two types of tissues.
Validating the new tool
For this preclinical validation, the team first confirmed the device’s ability to detect and locate inclusions in both a synthetic gelatin phantom and a swine-tissue model. The Raman spectral signature of the inclusions was distinct from the surrounding media, and the probe successfully directed “biopsies” from 20 suspect tissue areas, detecting inclusions and guiding the tissue excision. The only inclusions that were not accurately identified and excised were some of those smaller than 0.5 cc, considered to be the threshold for clinical significance.
Next, the team measured tissue samples from six prostate cancer patients and acquired 89 spectra at different locations. A pathologist then stained and evaluated the samples at those same locations. The probe system properly identified healthy or cancerous tissue in 81% of the cases.
The team performed their first in situ acquisition in a patient in September 2020. The cancer-detection ability of the tool can only be confirmed with a large number of patients, but initial results show that Raman spectral features can distinguish benign from cancer tissues. Kadoury feels this demonstrates that the device can improve clinical workflow, both for biopsies and to precisely guide radiation delivery in brachytherapy procedures. The probe could eventually replace biopsy altogether, eliminating both the risk of infection and the wait time for histopathological analysis. “The Raman detection system allows for real-time tissue characterization,” he says, “and if the sample comes back positive, the patient can be treated right away.”
About the Author
Richard Gaughan
Contributing Writer, BioOptics World
Richard Gaughan is the Owner of Mountain Optical Systems and a contributing writer for BioOptics World.