FLUORESCENCE: Taking life by STORM
Stochastic optical reconstruction microscopy (STORM) enables generation of 2D and 3D multicolor imaging of tissues and cells with near molecular-scale resolution.
By Stephen T. Ross
In the past few years, several breakthrough technologies have been introduced that break the traditional limits of optical microscopy. Of them, multicolor, three-dimensional stochastic optical reconstruction microscopy (STORM) is one of the most promising for observing tissues and cells with near molecular-scale resolution—and the technology is about to become available commercially (see Fig. 1).
STORM, developed in the lab of Xiaowei Zhuang—Howard Hughes Medical Institute Investigator, Professor of Chemistry and Chemical Biology, and Professor of Physics at Harvard University (Boston, MA), uses photo-switchable fluorescent probes to temporally separate the otherwise spatially overlapping images of individual molecules, allowing the construction of super-resolution imagery. Using this concept, two- and three-dimensional, multicolor fluorescence images of molecular complexes, cells and tissues with a few 10s of nanometers resolution has been achieved. This approach allows nanometer-scale imaging of molecular interactions in cells and cell-cell interactions in tissues. (See the sidebar, Approaches to super-resolution.)
What it means and how it works
Until the late 1980s, most life scientists investigated the intricate details of biological structures by capturing single snapshots of cytological features using fixed and stained specimens. More recently, the technique of imaging cells on the microscope stage evolved with the emergence of immuno-conjugated synthetic fluorophores and fluorescent proteins to serve as qualitative and quantitative reporters of intracellular structure and dynamics. Cellular imaging now spans multiple modalities, including widefield (fluorescence, phase contrast and differential interference contrast), laser scanning confocal, multiphoton, and spinning disk microscopy.
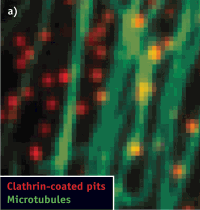
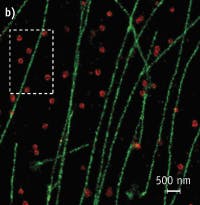
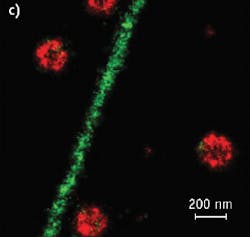
Figure 2. Conventional fluorescence (left) and STORM images (center, right) show the same region of microtubules in a mammalian cell. (Images adapted from Science, 319, 810-813 [2008].)
STORM reveals molecules' positions in all three dimensions—with lateral resolution to approximately 20 nm and axial resolution to approximately 50 nm—thus enabling scientists to gain new insights into the dynamic associations of co-localized proteins in cells at a scale an order of magnitude smaller than before.
STORM achieves its high image resolution by relying on the detection and localization of single fluorescent molecules (see Fig. 2). Due to the diffraction of light, the image of a single fluorescent molecule has a finite size of approximately 200–300 nm in a lateral direction and 500–800 nm along the axial direction. Nonetheless, the position of the molecule can be determined to a much higher precision by fitting the image to find its centroid position. The positioning precision depends on the number of photons (N) detected from the molecule. That is:
Precision = the width of the image / √N
For example, the position of a single dye molecule can be determined to accuracy as high as ~1 nm (see Fig. 3). This high-precision localization, however, does not directly give sub-diffraction image resolution, as the images of nearby molecules would be highly overlapping, making their positions difficult to determine. But STORM provides a solution.
STORM is based on the sequential imaging and localization of these molecules, which can be optically switched between a fluorescent state and a dark state. When the fluorescence emission from these molecules is controlled over time, researchers can map their positions. Specifically, in the imaging process, only a subset of molecules is activated to the fluorescent state at any given time (for example, by exposure to light of a certain wavelength and intensity), such that the images of individual activated molecules do not typically overlap. By fitting these isolated images, the positions of the activated molecules can be localized with high precision, as described above. This process is then repeated to allow more molecules to be localized. Once all the molecules, or a sufficiently large number of molecules, have been localized, a high-resolution image can be constructed from the measured positions of these molecules. Thus, the resolution of a STORM image is limited not by diffraction, but by the localization precision of the molecules.
A major advantage of fluorescence microscopy is the capacity for multicolor imaging, which enables visualization of the relative organization and interactions between different biological molecules. In multicolor STORM imaging, a family of photo-switchable "reporter" dyes, including Cy5, Cy5.5 and Cy7, can be cycled between a fluorescent and a dark state in reversible manner by exposure to light of different wavelengths. Red light can excite fluorescence and also cause the dyes to switch off. The reporter dyes can be efficiently reactivated when an "activator" dye is placed in close proximity if the wavelength of activation light matches the absorption peak of the activator. Not only can different pairs be distinguished by their emission color, as determined by the reporter dye, but they can also be differentiated by the color of light that activates them, as determined by the activator dye. Combinatorial pairing of reporters and activators allows the construction of a large number of spectrally distinguishable fluorescent probes and enables STORM imaging of cells with many colors.
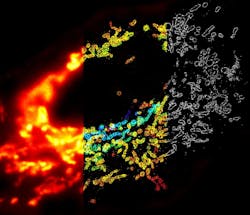
Figure 3. The z-position of microtubules in a cell is color-coded according to the colored scale bar. (Images adapted from Science, 319, 810-813 [2008].)
In December 2009, Harvard University signed a licensing agreement granting Nikon Corporation the rights to use the STORM technology. Under the agreement, Nikon has begun to manufacture and market N-STORM instrumentation, and plans to begin delivering systems in September 2010.
The potential of STORM technology will be shaped by those who are using it. These not only include biologists who adopt the STORM technology to study their specific biological systems, but also physicists and chemists who develop other ways of manipulating the imaging system to achieve novel imaging capabilities.
Importantly, the resolutions mentioned above do not represent the ultimate limit of STORM. Its spatial resolution is determined by the precision and density of localizations in the image. These two quantities are determined by four practical factors: the brightness of the probe, residual dark-state fluorescence, labeling efficiency and label size. Given sufficient probe brightness and labeling density, resolution can be almost arbitrarily high. For a bright probe, such as Alexa Fluor 647, the number of photons detected allows in principle a localization precision of a few nanometers, promising true molecular-scale resolution.
What researchers see next depends upon how scientists are able to adapt the STORM technology to their research and laboratories.
References
- Science Vol. 313. no. 5793, pp. 1642 – 1645 (2006)
- Nature 462, 675-678 (2009)
STEPHEN T. ROSS is Senior Manager, Bioscience Product and Technology, Nikon Instruments, Melville, NY, www.nikoninstruments.com; e-mail: [email protected].
Approaches to super-resolution
Of the various forms of super-resolution microscopy, photoactivated localization microscopy (PALM) is perhaps most like STORM. PALM takes multiple frames of photoactivatable proteins scattered throughout a sample and combines them into a high-resolution image. The technique is capable of producing lateral resolution to approximately 20 nm and axial resolution of about 100 nm, and is commercialized by Carl Zeiss (Oberkochen, Germany) as the ELYRA P.1.1
Three-dimensional structured-illumination microscopy (3D-SIM) illuminates a sample with a series of light patterns. The high spatial frequency patterns reflect off the fine structure of the sample to create moiré fringes. By applying the light patterns in different orientations and using algorithms to process the reflections, it is possible to produce high-resolution imagery.2 The approach is commercialized by Applied Precision (Issaquah, WA) as DeltaVision OMX, by Carl Zeiss as ELYRA S.1, and by Nikon as N-SIM; it approximately doubles the resolution of conventional optical microscopes and carries potential for more.
Stimulated emission depletion (STED) uses a laser beam to excite fluorescent dyes, or fluorophores, within the sample. As with a normal laser scanning microscope, the size of the excited spot determines the resolution of the microscope. To improve resolution and narrow the focus of the beam, STED uses an excitatory laser pulse, which is immediately followed by a ring-shaped depletion laser pulse to leave molecules in a smaller region in the center of the ring in an excited state. This allows resolution of structural details in the 100 nm range. Commercialized by Leica Microsystems (Wetzlar, Germany) as TCS STED CW, the excitatory and depletion laser beams are continuous and overlayed rather than pulsed.2—BG
More Brand Name Current Issue Articles
More Brand Name Archives Issue Articles