NEAR-INFRARED IMAGING/ONCOLOGY: NIR fluorescence images lymphatics to address clinical need
By Eva M. Sevick-Muraca and Caroline E. Fife
The lymphatics vasculature has been referred to as "the forgotten circulation," even though it is critical to fluid homeostasis, mediating immune response and lipid absorption. Compared to the blood vasculature, little is known about the function of the lymphatics: There is a dearth of options for noninvasively imaging the clear and colorless lymph flow.
How lymphatics works—and doesn't
As an example of the importance of the lymphatics, consider the body's mechanisms of normal wound repair. The body's ability to repair wounds or maintain fluid homeostasis critically upon an orchestrated balance between (i) the venous circulatory system that drains water and small proteins into a site of injury and (ii) the lymphatic circulatory system that transports macromolecules, cells, bacteria, and debris from sites of tissue injury and inflammation. When the lymphatic system goes awry for reasons not yet completely understood, a "back-up" of fluid or a "flood" occurs, causing tissue swelling. When there is irreversible swelling, the disease of lymphedema results. Recently, our collaborative team—at the University of Texas Health Science Center and the Texas Medical Center (Houston, TX)—built and deployed near-infared (NIR) fluorescence devices to image the lymphatic vasculature and its function in studies of patients suffering lymphedema.
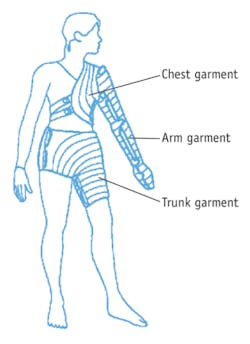
Lymphedema is an incurable disease that is often acquired in western countries following lymph node removal and/or radiation for cancer treatment. Lymphedema impacts an estimated 3–5 million of the 12 million cancer survivors alive in the U.S. and consists of irreversible swelling–for instance, of the arm in breast cancer or melanoma survivors, or leg in those treated for prostate or bladder cancer. If left untreated, lymphedema can be disfiguring and debilitating. The management of lymphedema critically depends upon stimulating lymphatic function through manual lymphatic drainage (MLD), pneumatic compression devices (PCDs; see Fig. 1), or a combination thereof.
Unfortunately, there are no diagnostic methods available to directly and quantitatively assess the efficacy of MLD, PCDs, and other emerging treatments to improve lymphatic function. Recently, the U.S. Centers for Medicare and Medicaid Services (CMS) challenged the adequacy of available evidence to support diagnostic and treatment methods currently used in lymphedema management,1 suggesting that continued reimbursement for treatment requires new evidence of benefit. Current diagnostic measures (including lymphoscintigraphy and limb volume measurements) and standards of clinical treatment (including MLD and several marketed PCDs) were deemed to have insufficient evidence of benefit.
Examining effectiveness
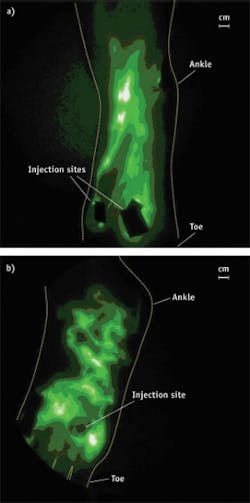
Recently, in FDA-approved studies, we demonstrated that investigational near-infrared (NIR) fluorescence lymphatic imaging in humans may enable diagnosis of aberrant lymphatic function, personalized evaluation of the efficacy of MLD and PCDs, as well as directing course of therapy—all at the point-of-care.3-7 The technique depends upon a 0.1 cc intradermal injection of indocyanine green (ICG), an agent that has been used for over 50 years for assessing hepatic clearance, cardiovascular function testing and retinal angiography on the basis of its dark green color. ICG is also a dim, NIR fluorescence dye, making it a feasible fluorescence imaging agent for assessing lymphatic function and protein transport. Following intradermal injection of ICG at 1,000 times less dose than currently approved for intravenous administration (termed a microdose when <100 micrograms by FDA definitions), we found the dye transits immediately into normal initial lymphatics and is actively transported to draining lymph node basins. Using a 785 nm laser diode with incident tissue fluences <1.9 mW cm2, tissue surfaces are dimly illuminated with NIR excitation light. The excitation light harmlessly penetrates several centimeters into tissues and activates ICG. The ICG fluoresces, and fluorescent light propagates to the tissue surface and is collected by a 16-bit frame transfer CCD camera outfitted with light rejection optics and a Gen III image intensifier.6 The imaging system provides large fields of view (and large working distances) while noninvasively imaging microdosage administrations of a poor fluorophore (ICG) streaming through the lymphatics with rapid image acquisition times. We were able to achieve a high value of sensitivity by using a monochromatic light source and optical filters carefully selected to reject the overwhelmingly large component of backscattered excitation light while selectively passing the fluorescence light to the detector.6,8Owing to the sensitivity of the imaging system, we acquire images on the order of 50–200 ms after microdosing of ICG and compiled "movies," depicting for the first time the "pumping," lymphatic propulsive function in humans.2 In our studies, we quantified lymphatic function in terms of a frequency of lymphatic propulsion.
Using NIR fluorescence lymphatic imaging in a cohort of normal subjects and subjects with lymphedema, we quantitatively assessed the improvement of lymphatic "pumping" and recruitment of new lymphatic vessels in response to the treatments of MLD7 and with a specific PCD.4 By evaluating lymphatic function and the movement of ICG through tissues both before and after therapy in a single treatment session, NIR lymphatic imaging was shown to potentially enable patient-specific care by indicating who will and will not benefit from treatment. Currently, patients must be treated over weeks and before benefit can be measured through a reduction of limb volume (typically assessed with a measuring tape), whereas NIR fluorescence imaging could enable personalized medicine by assessing the most efficacious treatment that stimulates lymph pumping. More importantly, NIR fluorescence imaging could provide the evidence to continue reimbursement of the only treatment approaches available to many cancer survivors.
Multiple benefits
It may not be surprising that NIR fluorescence imaging is more sensitive than lymphoscintigraphy, which depends upon the administration of radioactive colloid that, upon decay, releases a single gamma photon from each radionuclide for collection using a gamma camera. Several minutes or hours after radiocolloid administration, integration of photon counts over several minutes produces a low-resolution gamma image, sometimes depicting main lymphatic vessels and draining lymph nodes. Because gamma imaging requires significant integration time, the rapid contractile function responsible for lymphatic motion cannot be imaged as it can with NIR fluorescence imaging. The rapid acquisition and enhanced sensitivity of NIR fluorescence imaging depends upon the ability to repeatedly excite ICG with tissue-penetrating NIR excitation light. ICG (and other NIR excited fluorophores) can repeatedly be activated and emit fluorescent photons on the order of the fluorophore lifetime (typically <1 ns). Consequently, each dye molecule can theoretically emit as many as 100,000,000 NIR photons per second. Compared with the single gamma photon emitted from the radiocolloid, NIR fluorescence imaging offers enhanced sensitivity, improved resolution, lack of radioactivity and compact instrumentation that may facilitate point-of-care use. The current limitation to the sensitivity and depth of penetration of NIR fluorescence imaging depends upon the ability to selectively collect fluorescent photons while rejecting the overwhelmingly larger component of backscattered excitation light. Rejection of light is an exciting area for future improvement by optical engineers and physicists.
Given the high photon count rates demonstrated at this early stage of human application, the continued development of 3-D NIR fluorescence tomography remains promising, and the future use of brighter NIR fluorophores (enabling better imaging with smaller doses than ICG, but not yet tested in humans) will certainly improve lymph imaging quality and promise more facile discoveries in humans than possible with ICG.
We are now moving forward with better, brighter agents. A brighter fluorophore will also allow us to engage in 3-D tomography using time-dependent methods as we originally intended. By using time-dependent measurements (whether in the time- or frequency-domain), we have demonstrated the ability to reconstruct 3-D images from a single projection of 2-D fluorescence measurements. For human applications, this is what would be required since tissue penetration largely limits acquisition of 360 degrees of NIR projections. And all time-dependent techniques entail a deteriorating instrument response that increases the necessity of using brighter fluorophores and better excitation light rejection techniques.
The original goal of our research program was to use NIR fluorescence tomography to image the number of cancer positive lymph nodes in breast cancer patients without requiring the use of sentinel or axillary lymph node dissection. A non-invasive imaging approach with a molecularly targeting agent—able to specifically image cancer cells within lymph nodes—would eliminate surgical biopsies of lymph nodes and reduce the incidence of lymphedema.9, 10 Most importantly, if we could show the NIR imaging and tomography technology was comparable or superior to surgical axillary lymph node biopsy, then we could improve the accuracy in staging of breast cancers and improve survivorship.
Meanwhile, the current state of the NIR fluorescence imaging offers for the first time the ability to assess lymphatic function for effective management of lymphedema, and could in the future be used to justify the continued reimbursement of therapies for cancer survivors.
REFERENCES
1. M. Oremus, "Diagnosis and treatment of secondary lymphedema," Medicare Evidence Development & Coverage Advisory Committee meeting (2009)
2. J.C. Rasmussen et al., Current Opinion in Biotechnology 20, 1–9 (2009)
3. J. C. Rasmussen et al. Transl. Onol. 3 (6): 362–372 (2010)
4. K.E. Adams et al., Biomedical Optics Express 1, 114–125 (2010)
5. E.A. Maus et al., "Near-infrared fluorescence lymphatic imaging of head and neck lymphedema," to appear in Head and Neck.
6. M.V. Marshall et al, The Open Surgical Oncology Journal 2, 12–25 (2010)
7. I.C. Tan et al., "NIR fluorescence imaging of improved lymphatic propulsion and transport following manual lymphatic drainage," to appear in Archives of Physical Medicine and Rehabilitation.
8. B. Zhu et al., Medical Physics 37 (11): 5961–10 (2010)
9. A. Giuliano et al., JAMA 305 (6): 569–575 (2011)
10. D. Grady, "Lymph Node Study Shakes Pillar of Breast Cancer Care," The New York Times, February 8, 2011.
EVA M. SEVICK-MURACA is Professor and Cullen Chair in Molecular Medicine at the University of Texas Health Science Center's Institute of Molecular Medicine (IMM), where she directs the Center for Molecular Imaging. CAROLINE E. FIFE is a physician with the Division of Cardiology and Hyperbaric Medicine, Department of Internal Medicine at the University and with Memorial Hermann Center for Lymphedema Management at Texas Medical Center, Houston, TX. Contact Dr. Sevick-Muraca at [email protected].