Cancer is an all-too familiar killer, but institutes such as the NIH and countless privately funded companies are working for a cure. Fortunately, methods for accurate cancer diagnosis—which extend lives and improve quality of life for those lucky enough to find the disease early—are making full use of the latest optical and photonic innovations. Gold nanoshell-based markers are infiltrating cancer cells and lighting up these tissues in conjunction with multiple imaging modalities such as optical molecular imaging. Some nanoparticles provide a photothermal effect to not only identify cancer cells but to kill them at their source; yet other methods such as quantum-cascade laser-based absorption spectroscopy, which can illuminate tissue samples with enough spectral power density to potentially differentiate between different forms of cancer or precancerous tissue using “objective” spectroscopic trace results rather than “subjective” image review by a trained medical specialist.
At the cellular level
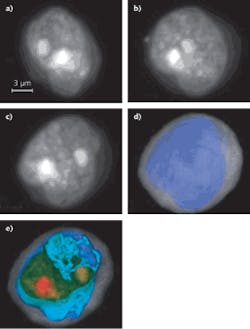
Commercially available to US research labs since early this year, the Cell-CT platform from VisionGate (Seattle, WA) can analyze any bodily fluid (sputum, urine, blood) with cellular content in suspension by computing the three-dimensional (3D) internal structure of cells based on stain absorption densities, marker color, and simultaneous optional fluorescence or any microscopy contrast mode. Cells are injected through a micro-capillary tube that is rotated to allow image collection around 360°. A 3D imaging reconstruction of stained (hematoxylin) cellular features and other absorbing structures (cytosplasm, nucleoplasm, and nucleoli) within a cell at submicron resolution is possible using a tomographic reconstruction and a VisionGate innovation called the pseudo-projection.
To gather pseudo-projection images, a standard Olympus IX71 microscope fitted with an oil-coupled 100X 1.3 numerical aperture (NA) objective is coupled to a Piezosystem Jena objective Z scan motor and a 2 Mpixel Prosilica GE1650 CCD camera. A 50 nm wide monochrome illumination source in the green spectrum contrast enhances specific stained cell features. The camera, a rotation mechanism, and the scanning lens are synchronized to collect as many as 500 pseudo-projection images per cell around 360° of rotation at a rate of 5 frames per second (fps). The Cell-CT’s 3D reconstructions provide physicians the means to detect cancer in high-risk patients at an early stage and to detect other cellular features that may progress to cancer as well as other lung conditions. In one recent experiment, the Cell-CT identified cancerous squamous cells in 75% of the sputum samples tested from lung-cancer patients (see Fig. 1).1
To bring the Cell-CT instrument from research into the clinical realm, VisionGate is using the latest cameras in high-speed photography from Vision Research to improve pseudo-projection capability to 1000 fps. The company is also marrying a traditional laser-based flow cytometry frontend with the Cell-CT, allowing cytologists to use the speed of standard flow cytometry and combine it with ultraspecific 3D analysis of cellular morphological marker position and stained features.
Going in vivo
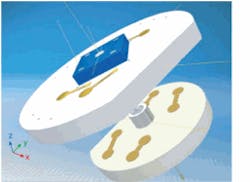
In vitro analysis of samples extracted from the body finds cancer typically at a late stage in its development, when the cancer has already metastasized. The next logical step is to perform cancer diagnosis in vivo using an optical fiber endoscope. Scientists at the Fraunhofer Institute for Photonic Microsystems (IPMS; Dresden, Germany) are developing an endoscopic sensor that speeds tumor detection (see Fig. 2).2 The probe is a microelectromechanical systems (MEMS)-based microscope only 8 mm in diameter that can image cells measuring just 30 to 50 µm in diameter.The endomicroscopy system delivers about 20 mW of 532 nm laser light through a single-mode optical fiber to a MEMS mirror at its tip. This light is deflected toward the tissue and the reflected beams are gathered to create an image that is then analyzed by a trained expert to see if cancer is present in the area being imaged. The size of this device makes it suitable for diagnosing gastronomical and tracheal cancers, as long as the opening is larger than the 8 mm diameter of the probe.
From subjective to objective
The unfortunate reality of cancer diagnosis is that many photonic and imaging methods can identify cancer cells only when the eye of a trained clinician analyzes the visual images. Block Engineering (Marlborough, MA) is trying to change that reality with its LaserScan Analyzer, a mid-infrared (mid-IR) laser-based spectrometer that aims to move “subjective” cancer analysis into the “objective” realm.
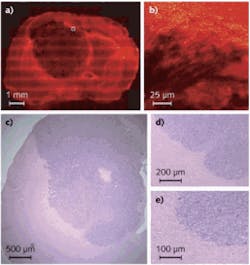
LaserScan uses IR absorption spectroscopy and a tunable quantum-cascade laser (QCL) source to rapidly scan over a 5 µm spectral region anywhere within its 6 to 12 µm wavelength operating range—an area of the spectrum known as the fingerprint region—that provides highly sensitive (and selective) spectral signatures for biological tissues such as prostate cancer with much greater power than a conventional Fourier-transform IR (FTIR) spectrometer. For example, Rohit Bhargava, assistant professor of bioengineering at the University of Illinois, Urbana–Champaign, is using a synchrotron IR source in combination with an FTIR microscope and computer algorithms to analyze spectroscopic images of tissue to develop classification schemes for IR chemical imaging of prostate and breast cancers.3 “LaserScan offers a much more compact alternative to a synchrotron and has the possibility of bringing this cancer detection approach from the scientific laboratory to the pathology lab,” says Adam Erlich, VP of marketing and business development with Block Engineering. “Its semiconductor laser source has two-orders-of-magnitude greater spectral power density and resolution than a standard FTIR microscope with a conventional IR source… providing good signal-to-noise ratio and improved spatial resolution and spectral quality, leading to more precise mapping of tissue samples—attributes that are absolutely imperative in determining whether a tissue is cancerous.”Also with the aim of “objective” cancer diagnosis (but acknowledging the value of a subjective, trained clinician), Harvard University (Cambridge, MA) graduate students Brian Saar and Christian Freudiger are working in the lab of Sunney Xie using coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS) to visualize the margins of cancerous brain tumors.4 The work is a collaborative effort with Dr. Geoff Young and colleagues at Brigham and Women’s Hospital. “By using CARS and SRS, we have observed spectroscopic differences between normal tissue and cancerous tissue based on the differences in lipid and protein density,” says Saar (see Fig. 4). “Many groups working on approaches to distinguish healthy tissue from cancerous tissue use point spectroscopy combined with mathematical models that pick out subtle spectral features to make this distinction. However, this is not our focus.” Instead, says Saar, Harvard is using a combination of chemical contrast due to Raman spectroscopy and rapid, high-resolution imaging. Because CARS and SRS offer high sensitivity compared to ordinary spontaneous Raman scattering, 3D images with submicron resolution can be acquired in tissue (even in vivo) at 30 fps or even faster (compared to hours per frame for spontaneous Raman scattering).
“Because coherent Raman techniques offer intrinsic label-free chemical contrast due to molecular vibrational frequencies and the 3D optical sectioning capability of multiphoton microscopy, we believe that we can offer pathologists images with the same diagnostic features that they currently use, except that in our case these will be instantly available from inside the patient using either an endoscope or surgical microscope,” adds Saar. “This will spare the patient the delays and potential adverse effects associated with brain biopsy and tissue sample preparation, potentially improving patient outcome. It allows us to leverage the ability of a trained pathologist to recognize subtle, microscale features of tissue that allow for exquisite sensitivity and specificity in diagnosis rather than replacing these features with a ‘black box’ mathematical model, which—from talking with our clinical colleagues—could be a major barrier to acceptance.”
From the clinician’s standpoint, photonic methods of cancer diagnosis are invaluable. “The number of possible diseases in an organ like the brain is enormous, with hundreds of commonly encountered diseases and tens of thousands of rare diseases,” says Dr. Young. “I am very excited about our approach: using advanced optical methods to produce immediate, nontoxic, minimally invasive diagnostic pathology images with chemical-, cell-, and tissue-specific contrast so that diagnostic pathologists can render rapid definitive diagnosis of the live patient before surgery rather than having to analyze tissues removed during surgery or at autopsy.”
Lighting up cancer
Another very “objective” method of diagnosing cancer is to engineer nanoparticles, dyes, and chromophores that penetrate or “stick” to cancer cells and light up for easy identification.
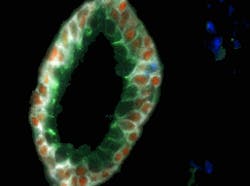
In the nanotechnology world, new research breakthroughs are occurring rapidly and many current news releases can be found at www.nano.cancer.gov. Just in May 2010, Emory University (Atlanta, GA) researchers were able to use multicolored quantum dots (QDs) conjugated to antibodies for molecular and cellular mapping of tumor homogeneity on human prostate cancer specimens.5 The fluorescent QDs (4–8 nm core diameter, fluorescing in wavelengths between 500–700 nm) were detected in tumor biopsies using a Nuance spectral imaging microscope from Cambridge Research & Instrumentation (CRi; Woburn, MA). The technique is so precise that it is able to show how prostate cancer likely starts with a single malignant cell in the luminal and basal layers of the prostate gland (see Fig. 5). Imagine how early a cancer diagnosis could be if this single cell could be identified in vivo.Nanotechnology research institute IMEC (Leuven, Belgium) recently developed asymmetric gold nanostructures that combine nanorings and nanodiscs and exhibit a change in spectral response upon binding with specific cancer markers in human blood. By exploiting surface plasmon resonance for these structures, the width of the resonance peak and the shift in resonance upon binding with the marker is identified more easily than with traditional nanosphere-based cancer diagnosis methods.
An alternate nanoparticle design from researchers at Purdue University (West Lafayette, IN) and Washington University (Saint Louis, MO) claims to yield images 10X brighter than other experimental results using gold nanospheres and nanorods and hundreds of times brighter than conventional fluorescent dyes.6 The hollow, 40 nm wide nanocages—made of an alloy of gold and silver—are illuminated by an NIR ultrafast (femtosecond) laser source that induces three-photon luminescence (as well as generating light via third harmonic generation, it is thought, for increased brightness). Because surface plasmon effects are not used, no heating is involved and background autofluorescence is not observed.
Going a step further, what if clinicians could eliminate the diagnosis step entirely and use nanoparticles that bind to cancer cells and kill them? In this case, nanoparticle heating may not be a disadvantage, as long as the particles bind only to cancer cells and not to healthy human tissue. A recent demonstration by Washington University researchers used a diode laser to illuminate cancerous tumors in mice that had been injected with 40 nm diameter gold nanocages.7 The tumors were destroyed after only a few minutes. And Hsien-Rong Tseng and his colleagues at the University of California–Los Angeles (UCLA) are creating self-assembling supramolecular gold nanoparticles bound to particular molecules that bind to cancerous tumor cells.8 When these 118 nm diameter particles are irradiated by a laser, the temperature soars above 374°C and explosive microbubbles form that kill the cancer cells while leaving healthy cells intact. “Like laser-triggered nanobombs, these self-assembled gold nanoparticles selectively kill cancer cells,” says Tseng. “In conjunction with a two-photon laser, these supramolecular gold nanoparticles could be used to overcome the tissue-penetration limits of cancer treatment in the clinic.”
REFERENCES
1. M.G. Meyer et al., “The Lung Cell Evaluation Device (LuCED): Early Detection of Lung Cancer in Sputum Based on 3D Morphology,” 13th World Conference on Lung Cancer, paper TBD, San Francisco, CA (Jul. 31–Aug. 4, 2009).
2. www.fraunhofer.de/en/press/research-news/2010/06/detecting-tumors-faster.jsp
3. C. Henry Arnaud, Chem. and Eng. News 86, 12, 56–58 (March 2008).
4. C.L. Evans et al., Opt. Exp. 15, 19, 12076–12087 (2007).
5. J. Liu et al., ACS Nano 4, 5, 2755–2765 (2010).
6. L. Tong et al., Angewandte Chemie Intl. Edition 49, 3485–3488 (2010).
7. J. Chen et al., Small 7, 811–817 (2010).
8. www.nano.cancer.gov/action/news/2010/may/nanotech_news_2010-05-21c.asp