Optofluidics: FRET optofluidic microlasers enhance biological sensing
YASIN KARADAG, ALEXANDR JONÁŠ, and ALPER KIRAZ
Biological molecules—proteins or nucleic acids—are commonly studied by tagging them with light-emitting fluorescent markers. When two different markers with suitable spectral profiles are linked to a single molecule of interest, fluorescence resonance energy transfer (FRET) between these markers can serve as a precise ruler for measuring distances within or between the molecules with atomic-scale resolution. Changes of intramolecular and intermolecular distances then directly report on the molecular activity triggered by controlled external stimuli.
Typically, the intensity of spontaneous FRET light emitted from the sample is detected and evaluated. However, the level of the spontaneous emission is often too low to collect a sufficient amount of photons. Amplified stimulated emission from the sample placed inside an optical cavity can lead to FRET lasing and, subsequently, a significant increase in the emission intensity. Because of the optical feedback provided by the cavity, a small molecular change induced by underlying biological processes is significantly amplified, leading to a dramatic change in the laser output characteristics.
In our research, optofluidic FRET lasers are obtained using liquid solutions of fluorescent dyes or quantum dots as the gain media placed inside a microcavity. Highly sensitive amplified stimulated emission in the microcavity with high quality (Q) factors leads to up to 100X enhancement in detection sensitivities. By exploiting material characteristics and geometrical shapes of liquids, we create miniaturized light sources embedded directly on a chip for optimizing biological sensing applications.
FRET-based biosensing
Reliable detection and identification of biologically relevant molecules present in solution in low concentrations, often masked by the background of other similar molecules, is a task of utmost importance in biomedical analysis, environmental monitoring, or fundamental research in cell and molecular biology.
To achieve the selectivity needed for discriminating between molecules with very similar structures, most state-of-the-art sensors are based on the recognition of the target analyte by its natural or artificially engineered molecular counterpart that binds the analyte with high affinity and specificity (e.g., antibody and antigen, ligand and receptor, or complementary DNA strands).1 Such specific interactions between the two binding partners can be visualized using nonradiative FRET between a pair of donor and acceptor dye molecules displaying sufficient spectral overlap that are attached to one or both binding partners.
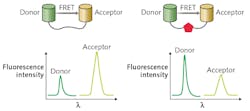
When biomolecules labeled with the donor and acceptor dyes undergo a conformational change or binding/unbinding process, the distance between the donor and acceptor changes, resulting in a change of the emission intensity of optically pumped donor and acceptor that constitute the FRET sensing signal (see Fig. 1).While FRET can provide information on molecular-scale processes, quantitative changes of the spectral profile of spontaneous FRET emission are usually small (on the order of a few percent). Consequently, signal-to-noise ratio of the traditional FRET-based detection is rather low, making reliable readout of the sensing signal challenging.
Recently, optofluidic lasers have emerged as a promising alternative, remediating the problem of noisy signal detection in conventional FRET assays.2 Optofluidic lasers integrate laser gain material and optical microcavities into liquid environments contained within microfluidic chips. Analytes under study then form the laser active medium located directly within the cavity. Thus, amplified lasing emission rather than ordinary spontaneous fluorescence serves as the sensing signal. Because of the optical feedback provided by the cavity, a small change in the gain medium induced by underlying intermolecular interactions is significantly amplified, leading to a dramatic change in the laser output characteristics.3-4
A biosensor based on optofluidic FRET lasing can be constructed by placing the studied analyte complex labeled with a suitable FRET acceptor-donor pair inside a laser cavity or, more precisely, within the cavity mode volume. Changes in FRET efficiency brought about by modulation of the separation distance between the donor and acceptor molecules will then translate into modulation of the laser output energy.
Recent research has demonstrated that the same change of FRET efficiency can result in changes in the lasing emission intensity from the donor and/or the acceptor that are orders of magnitude larger than those observed in conventional FRET based on spontaneous fluorescence.
Furthermore, besides intensity and polarization of the emitted light, laser output consists of additional parameters that can be monitored and serve as a measurement signal, such as the lasing threshold, lasing efficiency, and spatial profile of the lasing mode. Therefore, optofluidic FRET lasers provide a powerful complementary technology to analyze minute changes and interactions in biomolecules that may otherwise remain indistinguishable in the background noise.
Selecting the optimal cavity
Various experimental strategies for creating optical microcavities for integrated optofluidic lasers have been proposed and tested to date. Most frequently, the cavity is formed from a solid material. Examples of this approach include miniaturized versions of conventional Fabry-Perot cavities, distributed-feedback grating resonators, and optofluidic ring resonators (OFRRs) based on thin-wall capillaries that can support whispering gallery modes (WGMs)—spectrally narrow optical resonances with extremely high Q-factors of around 107 and small modal volumes localized in the proximity of the resonator surface.
The OFRR geometry is particularly well suited for optofluidic sensing applications, as it allows fast and easy exchange of the working fluid within the cavity. As a result, it is possible to quickly analyze different samples using the same experimental system. Furthermore, high-Q OFRRs facilitate the observation of lasing at comparatively low pump powers.
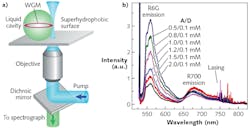
While all the above-described solid optical microcavities are fully functional, they require microfabrication and/or alignment steps that bring additional experimental challenges to the process of creating a working optofluidic sensor. In the simplest possible scheme, liquid alone—without any additional external solid structures—can be used to build the resonator. This is possible because of the tendency of liquids to minimize their surface area by transforming into perfectly spherical droplets. Liquid droplets with a refractive index higher than that of the surrounding medium (either air or another liquid immiscible with the droplet liquid) can then serve as alternative self-assembled ring resonators hosting high-Q WGMs.5
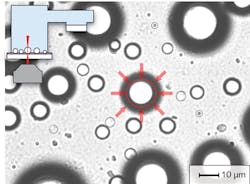
In contrast to OFRRs where WGMs residing inside the capillary wall interact with the analyte only through evanescent mode coupling, analytes can be placed directly into the cavity of a liquid droplet resonator. In this scheme, the analyte molecules that are located near the droplet surface can interact with the peak WGM fields, achieving very strong light-matter coupling. Moreover, aqueous microdroplets provide natural environmental conditions for biological molecules and live cells, making droplet cavities an ideal platform for sensing of biological species.
Despite these benefits, droplet-based cavities have found limited use in practical sensing applications to date-mostly because of difficulties with controlling and stabilizing the size and position of the droplets. However, as shown in our experimental work, the problem of droplet stability can be addressed by deposition of aqueous aerosol droplets on a superhydrophobic surface in an environment with controlled relative humidity or by suspending the droplets in an immiscible host liquid with a sufficient contrast of refractive index and confining them with the use of optical trapping techniques (see Fig. 2). The latter strategy is particularly amenable to integrating droplet-based cavities into lab-on-a-chip analytical and preparative microsystems.Fluorescent dyes or quantum dots?
Gain media formed by the analyte/receptor complex of interest linked to suitable chromophores (the "color-carrying" markers) that serve as the FRET acceptor and donor pair represent the crucial component of an optofluidic biosensor based on FRET lasing.
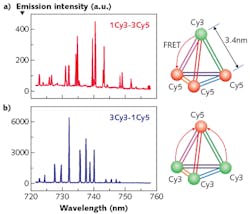
Fluorescent dyes are the most straightforward choice for selecting the FRET pair, as they can be attached covalently to the analyte and/or its receptor without disturbing the native complex conformation, have large emission cross-section and quantum yield of fluorescence, and are widely available as both polar molecules (suitable for aqueous environments) and nonpolar molecules (suitable for oil-like environments), covering the whole visible spectral range (see Fig. 3). And because of their high quantum yield, fluorescence-based gain media do not require resonant enhancement of the excitation light, which would have to be achieved by careful matching of the cavity size to the wavelength of the optical pump beam.A disadvantage of fluorescent dyes, however, is that they are inherently vulnerable to chemical damage because of light-induced bleaching, which alters the molecule such that eventually it becomes unable to fluoresce. Quantum dots, which can absorb and emit several-orders-of-magnitude-more photons before bleaching effects begin, can help overcome this limitation and allow for significant extension of the sensor operating lifetime.
Because of their high light-absorption capability in the blue and ultraviolet spectral regions, quantum dots can collect excitation light more efficiently, resulting in a significantly lower lasing threshold. Moreover, quantum dots emitting in different spectral regions can be readily excited using a single pump beam, allowing for parallel sensing of several analytes.
The recent demonstration of lasing in an aqueous optofluidic FRET system using quantum dots as the donor and Cy5 fluorescent dye as the acceptor in the FRET pair with incident pump fluence as low as 14 μJ/mm2 is an important step on the route towards a practical biosensing platform (see Fig. 4).6Although optofluidic FRET biosensors are still in an early stage of development, the fundamental proofs-of-principle have already been demonstrated, bringing them closer to practical applications in hospitals, environmental-monitoring stations, and for countless other applications as optofluidic technology continues to advance.
REFERENCES
1. A. Constantinou and K. M. Polizzi, Biochem. Soc. T., 41, 1146–1151 (2013).
2. X. Fan and S.-H. Yun, Nat. Methods, 11, 141–147 (2014).
3. E. Özelci et al., Laser Phys. Lett., 11, 045802 (2014).
4. M. Aas et al., Eur. Phys. J. – Spec. Top., 223, 2057–2062 (2014).
5. M. Aas et al., Opt. Commun., 290, 183–187 (2013).
6. Q. Chen et al., Lab Chip, 16, 353–359 (2016).
Yasin Karadag is an assistant professor in the Department of Physics at Marmara University, Istanbul, Turkey; e-mail: [email protected]; www.marmara.edu.tr; Alexandr Jonáš is an assistant professor in the Department of Physics at Istanbul Technical University, Istanbul, Turkey; e-mail: [email protected]; www.itu.edu.tr/en; and Alper Kiraz is a full professor in the Departments of Physics and Electrical and Electronics Engineering and principal investigator in the Optofluidics and Nano-Optics Lab at Koç University, Sarıyer/Istanbul, Turkey; e-mail: [email protected]; http://kirazlab.ku.edu.tr.