Mid-IR Sensing: Monolithic DFB QCL array aims at handheld IR spectral analysis
MARK F. WITINSKI, ROMAIN BLANCHARD, CHRISTIAN PFLUEGL, LAURENT DIEHL, BIAO LI, BENJAMIN PANCY, DARYOOSH VAKHSHOORI, and FEDERICO CAPASSO
Advances in infrared (IR) laser sources, optics, and detectors promise major new advances in areas of chemical analysis such as trace-gas monitoring, IR microscopy, industrial safety, and security. One key type of photonic device that has yet to reach its full potential is a truly portable noncontact (stand-off), chemically versatile analyzer for fast Fourier-transform infrared (FTIR)-quality spectral examination of nearly any condensed-phase material.
The unique challenges of stand-off IR spectroscopy actually extend beyond advances in IR hardware, requiring the proper combination of several areas of expertise: cutting-edge optical design and laser fabrication, integrated laser electronics, thermally efficient hermetic packaging, statistical signal processing methods, and deep chemical knowledge.
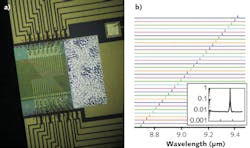
At the core of the approach we have taken at Pendar Technologies is the monolithic distributed feedback (DFB) quantum-cascade laser (QCL) array. Invented in Federico Capasso's group at Harvard University (Cambridge, MA) and licensed exclusively to Pendar, the continuously wavelength-tunable QCL array source is a highly stable broadband source that can be used for illumination in reflectance spectroscopy. Each element of the array is individually addressable and emits at a different wavelength by design.
The advantages of these QCL arrays over external-cavity (EC) QCLs stem from (1) the monolithic structure of QCL arrays and (2) their fully electronic wavelength tuning—that is, no moving gratings, allowing for much-higher-speed acquisition through improved amplitude and wavelength stability. When integrated into a system, the result is robust, stable, and field-deployable.
One of the key advances that has enabled this technology to be fielded is the high-yield fabrication of each laser ridge in the QCL array from a single wafer such that every channel simultaneously meets the specified wavelength, power, and single-mode suppression ratio. Each of these parameters is critical to both efficient beam combining and to obtaining high-quality molecular spectroscopy once integrated.
With these hurdles largely overcome, the payoff in terms of spectrometer performance lies largely in a demonstrated shot-to-shot amplitude stability in pulsed mode of <0.1%—a factor of 50 more stable than is typical for EC QCLs, even when used in the lab. Most importantly, the DFB QCL noise is random, and averages toward an Allan variance limit quickly such that detector-noise-limited, high-quality spectra can be obtained for trace levels (for example, 1–50 μg/cm2) of typical powders in just 100 ms.
More DFB array advantages
While the stability advantage of DFBs vs. EC configurations has been well established, there are a few less-obvious aspects to DFB arrays that make them more suitable to real-world spectroscopy tools and, in particular, portable spectroscopy tools. For one, the laser array as a whole can maintain a 100% duty cycle while each laser in the array requires operation only over a 100/n (%) duty cycle, where n is the number of lasers in the array. Put another way, a laser array consisting of only pulsed QCLs can operate as a truly continuous-wave (CW) system, allowing for high-measurement duty cycle while possibly reducing the cost of fabrication.
In a related way, generating light for an array that has a 100% aggregate duty cycle (by using, for instance, 32 lasers at 3% duty cycle), the thermal heat-sinking requirements of the source are dramatically reduced. Indeed, our packaged prototypes do not even require active cooling to keep the system cool enough to run. A thermoelectric cooler is built into the package only to stabilize the temperature, which therefore stabilizes the 32 wavelengths (see Fig. 1).
Finally, the arbitrary programmability of the QCL array opens up many new possibilities for experimental optimization. Certain lasers can be skipped, multiple lasers can fire at once, repetition rates and pulse durations can be set for each element, and so on. These advantages are only truly realized when the QCL array is instrumented into a full system.
Looking holistically at how best to integrate this new capability into a full system, it is critical to draft the link equations that govern the use of electrons to produce photons, the collection of photons scattered back, and finally the conversion from raw spectral information to chemical identification. In the case of mid-IR material identification, it becomes clear that three aspects are particularly consequential: (1) How broad a wavelength range is needed for the tool to be of maximum specificity without producing redundant or useless chemical information (that is, how many laser channels should be used, how should they be spaced with respect to one another, and over what total wavelength regime should they be spaced); (2) the mechanical and electro-optical design of the instrument; and (3) how to get the highest performance regressions against reference spectra while maintaining the high-speed identification that the QCL array actually enables.
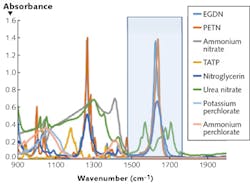
With regard to the wavelength regions of interest (see Fig. 2), most of the spectral richness of an IR spectrum is centered in two bands, generally referred to as the functional group region (about 3.3–5.5 μm) and the fingerprint region (about 7–11 μm). The first is typically dominated by the stretch modes of certain common bond groups, while the latter includes bending modes of some functional groups as well as lower frequency modes that are characteristic of the macromolecule "backbone"—for instance, the torsional modes of a toluene ring found in many highly energetic materials. With support from the Department of Homeland Security (DHS)'s Widely Tunable Infrared Source (WTIRS) program and from the Army Research Lab, Pendar is developing a compact array module that fully covers 7–11 μm (900–1430 cm-1).System architecture drivers
To maximize signal-to-noise (SNR) while minimizing the required acquisition time, the system architecture is driven by the following first-order considerations:
1. Increasing the laser power enabled by relaxed thermal constraints as the heat load is distributed over several modules (arrays) and laser waveguides.
2. Maximization of the measurement duty cycle enabled by the fast purely electronic control of the array, allowing close to zero-delay switching between lasers—that is, a laser is on at any time. This is also enabled by the distributed heat load among the laser units.
3. Improved source stability, wavelength accuracy, pulse-to-pulse amplitude, and frequency repeatability—all of which are needed to ensure that the source noise is not the limiting form of noise (compared to detector or speckle noise). Other researchers have studied the source-noise problem of commercial EC QCLs as well and concluded that the order-of-magnitude advantage in minimum detectable absorbance (MDA) offered by a DFB QCL carries through the full experiment.
Finally, once the spectra are digitized, the system must use complex chemometrics algorithms to ensure confident identification of threats in the presence of chemical clutter, deliberate interferents, and unknown backgrounds, without the intervention of an expert user. Our approach to real-time chemometrics is centered on the fact that for chemically cluttered situations, spectral libraries alone—no matter how large—cannot constitute the sole basis for chemometric analysis. Microphysics modeling and experimentation are also required, particularly in regard to crystal size distribution, clutter interactions, and chemical photolysis/reactions. The key advance lies in the incorporation of chemical and physical understanding of the targets and their co-indicators. We are currently developing a four-tiered approach to the spectroscopic algorithms challenge:
1. Physics-based models. Reliable chemical detection from standoff measurements will involve transformation of the chemical signatures in the reference spectral library to reflect the physical and environmental conditions of the experiment. A physics-based model will thus be included in the detection algorithm to help us model the variability in a reference spectrum as a function of effects such as vapor pressure, deliquescence, photochemical lifetime, reactive lifetime, decomposition products, and so on to facilitate better comparison with the measured spectrum.
2. Situational effects. Effects of different substrates and their properties on the chemical signatures and the angular dependence of spectra that are not clearly linked to equations of physics and chemistry will be experimentally evaluated and included in the detection algorithm. In particular, experimentally measuring such variability will help us algorithmically model the variability of chemical signatures from some "gold standard" reference signature, which—in addition to the physical model—will enable better detection strategies.
3. Feature-based classification. Extraction of relevant feature vectors from the reference library spectra and the knowledge of the chemistry to form a hierarchical decision tree that will help us provide different levels of classification based on the customer requirements. For instance, if a customer is only interested in finding out whether a given chemical is an explosive, then we might save on computational cost by avoiding searching through the leaves of the decision tree to find out the exact chemical.
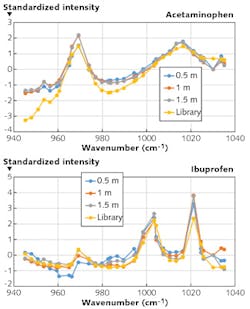
4. Real-time atmospheric measurements. Once validated, the model will be suitable for field implementation by the inclusion of an integrated sensor suite that simultaneously records atmospheric pressure, temperature, relative humidity, solar flux, wind magnitude, and water-vapor mixing ratio.With these design drivers considered, Pendar recently completed the build of a handheld demonstration system. Figure 3 shows the experimentally obtained spectra for two nonhazardous chemical targets as a function of stand-off distance. The yellow line in each panel shows the library FTIR ("true") spectrum for each. Agreements of r2 > 0.9 were typical. With the prototype system as an extrapolation point, continued, focused advances in the technology are now underway to open myriad frontiers in molecular spectroscopy.
ACKNOWLEDGEMENT
Pendar Technologies was formed in August 2015 through a merger between Pendar Medical (Cambridge, MA), a portable spectroscopy company founded by Daryoosh Vakhshoori (who was previously at Ahura Scientific and CoreTek), and QCL sensing startup Eos Photonics (Cambridge, MA), a Harvard spinoff founded by professor Federico Capasso and his postdocs.
Mark F. Witinski is vice president of the Chemical Analysis and Security Unit, Romain Blanchard is a systems architect, Biao Li is a senior scientist, Christian Pfluegl is vice president of engineering for the Infrared Systems Unit, Laurent Diehl is vice president of the Mid Infrared Platform, Benjamin Pancy is an optical engineer, Daryoosh Vakhshoori is chief executive officer, and Federico Capasso is a board member and advisor at Pendar Technologies, Cambridge, MA; e-mail: [email protected], www.pendartechnologies.com. Capasso is also a professor of applied physics at Harvard University, Cambridge, MA.