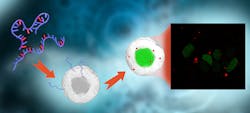
Fluorescence allows more efficient tracking of RNA, mRNA
Researchers at the Chalmers University of Technology (Gothenburg, Sweden) have developed a new method for labeling mRNA molecules, allowing them to follow cells’ paths in real time without any effect on their properties or activities.
According to the team, RNA-based therapies “offer a range of new opportunities to prevent, treat, and potentially cure diseases.” Currently, the delivery of RNA therapeutics in cells is inefficient—the new technique could ultimately boost that efficiency, and subsequently the development of RNA-based medicines and treatments.
“Since our method can help solve one of the biggest problems for drug discovery and development, we see that this research can facilitate a paradigm shift from traditional drugs to RNA-based therapeutics,” says lead researcher Marcus Wilhelmsson, a professor in Chalmers’ Department of Chemistry and Chemical Engineering. He notes that delivery methods must be optimized for new therapeutics to fulfil their potential.
In the study, the researchers replaced one of the building blocks of RNA with a fluorescent variant, which was shown to maintain the natural properties of the original base.1 The fluorescent units were developed “with the help of a special chemistry” that the researchers say was then used to produce messenger RNA (mRNA), without affecting its ability to be translated into a protein at natural speed. In turn, that fluorescence allows researchers (with a microscope) to follow functional mRNA molecules in real time as they are delivered into cells (see figure).
“Until now, it has not been possible to measure the natural rate and efficiency with which RNA acts in the cell. This means that you get the wrong answers to the questions you ask when trying to develop a new drug,” Wilhelmsson says. Drug discovery becomes difficult, for example, when the researcher aims to find out at what rate a process takes place, but the method provides an answer that is only one-fifth of what is correct.
The research findings address challenges that exist with mRNA—that the molecules are very large and very charged while simultaneously very fragile. As a result of that, those molecules cannot enter cells directly and must instead be packaged. The team’s new technique uses very small droplets (lipid nanoparticles) to encapsulate the mRNA.
“The great benefit of this method is that we can now easily see where in the cell the delivered mRNA goes, and in which cells the protein is formed, without losing RNA's natural protein-translating ability,” says researcher Elin Esbjörner, an associate professor in the Chalmers Department for Biology and Biotechnology.
Real-time monitoring of the process and how the lipid nanoparticles and mRNA molecules are distributed throughout the cell after they are delivered will be crucial to addressing existing ineffectiveness. In turn, more efficient lipid nanoparticles can be developed; this is work the Chalmers team is now in the midst of.
The Royal Swedish Academy of Engineering Sciences has highlighted the Chalmers team’s work as “particularly important for increasing societal resilience to crises.”
REFERENCE
1. T. Baladi et al., J. Am. Chem. Soc., 143, 14, 5413–5424 (2021); doi:10.1021/jacs.1c00014.
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.