Light microscopy method discovers that banked blood loses functionality over time
Researchers at the University of Illinois at Urbana-Champaign (U. of I.) used a light microscopy technique to measure the stiffness of the membrane surrounding red blood cells over time. With the technique, they found that even though the cells retain their shape and hemoglobin content, the membranes get stiffer and therefore decrease the cells' functionality. The researchers' work has positive implications for blood stored in blood banks, as the technique could someday be used to monitor blood prior to administration to patients.
Related: Label-free, pulsed laser-driven imaging tool tracks nanotubes in cells, blood
The established "shelf life" for blood in blood banks is 42 days. During that time, a lot of changes can happen to the blood cells--they can become damaged or rupture. But much of the blood keeps its shape and, by all appearances, looks like it did the day it was donated. Realizing this, electrical and computer engineering professor Gabriel Popescu and his research team wanted to quantitatively measure blood cells over time to see what changed and what stayed the same to help determine what effect older blood could have on a patient. They used spatial light interference microscopy (SLIM), a technique developed in Popescu's lab in 2011, to noninvasively measure cell mass and topology with nanoscale accuracy. Through software and hardware advances, the SLIM system today acquires images almost 100 times faster than three years ago.
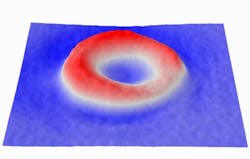
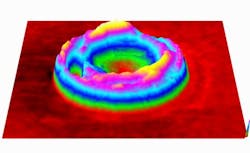
The researchers took time-lapse images of the cells, measuring and charting the cell's properties. In particular, they were able to measure nanometer-scale motions of the cell membrane, which are indicative of the cell's stiffness and function. The fainter the membrane motion, the less functional the cell, much like how a fainter pulse indicates problems with a patient.
Their measurements revealed that the cells retain their shape, mass, and hemoglobin content, but the membranes become stiffer and less elastic as time goes by. This is important because the blood cells need to be flexible enough to travel through tiny capillaries and permeable enough for oxygen to pass through.
"In microcirculation such as that in the brain, cells need to squeeze though very narrow capillaries to carry oxygen," says postdoctoral researcher Basanta Bhaduri, the lead author of the paper. "If they are not deformable enough, the oxygen transport is impeded to that particular organ and major clinical problems may arise. This is the reason why new red blood cells are produced continuously by the bone marrow, such that no cells older than 100 days or so exist in our circulation."
The researchers hope that the SLIM imaging method will be used clinically to monitor stored blood before it is given to patients, since conventional white-light microscopes can be easily adapted for SLIM with a few extra components.
Another clinical application for the technique, explains co-author Krishna Tangella, U. of I professor of pathology who is also affiliated with the Christie Clinic in Urbana, IL, could be using functional data from red blood cells to help physicians determine when to give red-cell transfusions for patients with anemia.
The research team's results appear in the journal Scientific Reports; for more information, please visit http://dx.doi.org/10.1038/srep06211.
-----
Don't miss Strategies in Biophotonics, a conference and exhibition dedicated to development and commercialization of bio-optics and biophotonics technologies!
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!