PROTEOMICS/BIOIMAGING: Ultrashort pulses of free-electron laser reveal nanoscale protein structure
Most of our knowledge of molecules' 3D structure has come from X-ray crystallography, a technique not well suited for life sciences because it involves and requires large crystals, which are scarce and inherently unstable in the bio realm.
But researchers recently demonstrated the use of ultrashort light pulses from the world's first hard X-ray free-electron laser to analyze protein crystals. Researchers from the Max Planck Institute for Medical Research (MPI; Heidelberg, Germany) and the Max Planck Advanced Study Group (Hamburg, Germany) used the U.S. Department of Energy's Linac Coherent Light Source (LCLS) at Stanford University (Stanford, CA) to reveal protein details to a resolution of 0.2 nm. Though the process destroys the crystals it analyzes, the pulses can pass through the specimen before the onset of detectable damage and thus provide the necessary scattering signal of the molecules while they are still intact. The approach produces data that compares well with those collected from large, well characterized crystals using conventional X-ray sources.
The diffraction-before-destruction approach involves injecting crystals into the free-electron laser beam using a liquid jet developed by scientists from Arizona State University (Tempe, AZ). Instead of rotating a single large crystal as in conventional crystallography, this method exposes one crystal after another. This concept of serial femtosecond crystallography has been demonstrated before by the same team of researchers at the Linac Coherent Light Source, using the CAMP instrument, which was developed by the Max Planck Advanced Study Group. The relatively long wavelength X-rays then limited the attainable level of structural detail.
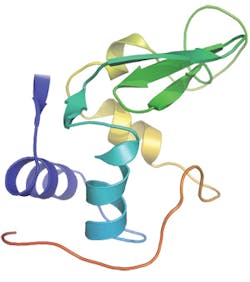
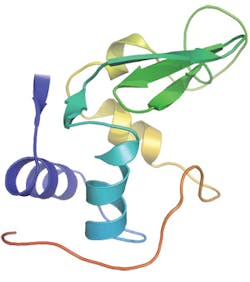
Recently, a new instrument at the Linac Coherent Light Source, the Coherent X-ray Imaging endstation, has allowed the use of short wavelength X-rays and thus made it possible to infer atomic detail in the molecular architecture. To benchmark the method, the team investigated the small protein lysozyme. The work is relevant for all studies of molecules that are difficult to crystallize-including 60 percent of all proteins, many of which are prime targets for medical therapies.
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles