Three-dimensional (3D) imaging is changing biology—and changing the world of drug development. This truth becomes clear in talking with 3D imaging specialists like Jacob G. Tesdorpf, director of high-content instruments and applications for life sciences and technologies at PerkinElmer's facility in Hamburg, Germany. For Tesdorpf, biology intertwines with drug discovery, and he sees 3D imaging used here in two ways. A scientist can look at a single cell or something made of many cells, from a cell culture to a small model organism, like a zebrafish. "If you image in 3D," he explains, "you can tell the spatial relationship of the components, regardless of scale." For example, two structures might appear side by side in two dimensions, but 3D could reveal that they actually lie far apart—at least in terms of cellular space.
Even cells themselves 'know' that 3D is better. That is, cells live in 3D environments in the body. As a result, 3D culture tells scientists more about how cells really behave. As Tesdorpf says, "3D cell culture is a better proxy for how cells really function and how they interact with neighboring cells." To see those 3D effects in action, scientists need tools that provide 3D images.
Tracking multiple targets
In drug development, a scientist might want to track more than one target—say, a couple of proteins—in 3D, and look for interactions between those targets. To get the most from drug-development experiments, that researcher must quantify the imaging results.
The recent Opera PhenixHigh Content Screening System from PerkinElmer provides a high-throughput approach to 3D confocal imaging to track fluorescent targets in microplates. This platform uses a scientific CMOS (sCMOS) camera that captures larger structures—three times larger than the platform that it updates—in a single image (see Fig. 1). A scientist can even deck out this system with four cameras to get simultaneous images from four fluorophores. Moreover, this system uses objectives from Carl Zeiss (Oberkochen, Germany), which provide large apertures and water immersion. "With water-immersion there's not as much change in the refractive index," says Tesdorpf, "so the resolution remains high as you move deeper into a sample." How deep, though, really depends on the sample at hand. Here, 'deep' might only mean 80 μm, but that gets paired with the ability to quickly explore many samples.
Matching modalities
In addition to tracking multiple targets, some medical researchers want to image a sample in more than one way. For example, the new InSyTe series from TriFoil Imaging (Chatsworth, CA) allows drug developers to combine optical imaging with other modalities, such as positron emission tomography (PET) and computed tomography (CT). As TriFoil CEO Kevin Parnham explains, "If you can image a subject in vivo, you can watch the progress of a treatment or the efficacy of a drug. To have any idea of the volume that you're looking at, you need to image in 3D." With the InSyTe system, a researcher can label a chemical in the body and then see where it gets taken up in a sample.
Some scientists will desire different combinations of modalities than others. To accommodate such a variety of uses, the InSyTe comes with a single modality or as many as three. It can also be upgraded later if needed. The optical modality provides 3D fluorescent imaging. The system's laser rotates around the sample and captures image information at 49 positions. For depth, the system samples in 1 mm steps. The system's software combines the images to build a 3D representation of the sample. Moreover, the system accommodates organisms from neonatal mice to small rats, up to about 300 g for the latter.
Although the InSyTe provides advanced imaging modalities, it does not require a highly controlled environment for use. Scientists can place the InSyTe on a benchtop in an ordinary lab. "It can tolerate a wide range of temperatures," Parnham explains.
Creating combinations
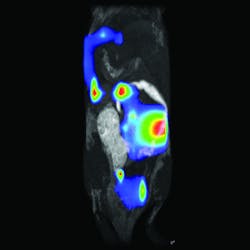
A combination of hardware and software makes up the LumiQuant from Aspect Imaging (Toronto, ON, Canada). This system consists primarily of a miniaturized magnetic resonance imaging (MRI) platform, which can be used without the cooling and shielded rooms needed for larger superconducting MRI systems. The LumiQuant integrates with leading luminescence platforms, including the IVIS technology from PerkinElmer, to bring in optical capabilities. For example, the LumiQuant allows MRI plus bioluminescent imaging (see Fig. 2). "The combination of hardware and software in the LumiQuant," says Robert Sandler, senior VP of marketing, "allows us to look at an optical bioluminescent signal localized in 3D with exquisite morphological reference from the compact MRI."LumiQuant collects an MRI and optical signal from the same sample by using a cassette. A mouse, for example, can be anesthetized, placed in the cassette, imaged with compact MRI, and then transferred to an optical system for bioluminescent imaging. "We use the MRI image for 3D morphological imaging, and then apply organ-specific filters—from an atlas—to the bioluminescence to detect elements of a disease at the molecular level and with organ-specific attenuation factors," Sandler explains.
Although the LumiQuant system provides high-powered imaging options, users do not need extensive training to use it. "The optical platform is literally push-button technology," Sandler says. "The compact MRI side is a little more complicated, but our users are usually biologists and not MRI experts." He adds, "With only marginal training, biology researchers and students can generate and quantify images on the Aspect Imaging m3 compact MRI platform."
Turning high-tech imaging capabilities into far more user-friendly tools will surely entice more basic researchers and industrial scientists to go 3D in medical research. By collecting an added dimension in drug research, scientists will get a better understanding of disease mechanisms, find safer and more effective medicines and, thereby, create new treatments that go far beyond today's medical options.
About the Author
Mike May
Contributing Editor, BioOptics World
Mike May writes about instrumentation design and application for BioOptics World. He earned his Ph.D. in neurobiology and behavior from Cornell University and is a member of Sigma Xi: The Scientific Research Society. He has written two books and scores of articles in the field of biomedicine.