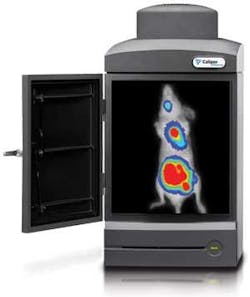
OPTICAL MOLECULAR IMAGING: In vivo commercial systems heighten appeal of molecular imaging
Last November, the Cleveland Clinic (Cleveland, OH) ranked an optical molecular imaging system as one of the year’s top ten medical innovations. “We believe this technology to be a game changer,” said Jennifer Hunt, the clinic’s head of surgical pathology. “When we’re talking about tumors, we’re talking about what information we can gain about that tumor to guide and direct therapy, prognosis, and diagnostics,” she said, referring to the clinic’s use of the Nuance system by Cambridge Research & Instrumentation, Inc. (CRi; Woburn, MA). “Being able to analyze multiple markers in a single cell to understand the behavior of signaling pathways will significantly aid in disease diagnosis and therapy development.”
While the first big application for in-vivo optical molecular imaging was infectious disease, oncology has been an important next step according to Caliper Life Sciences’ (Hopkinton, MA) Stephen Oldfield PhD. Indeed, Carestream Health Molecular Imaging (Rochester, NY) reports a surge of interest from oncologists just in the past couple of years. William McLaughlin, Director of Research and Advanced Applications for Carestream, says that at the American Association for Cancer Research (AACR) annual meeting two years ago, he saw significantly more interest in analytical techniques such as gel documentation and western blotting–but in 2008 noticed that more people were asking about the newer technology. Then at this year’s AACR meeting (April 18-22, Denver, CO), the majority of leads were for in vivo imaging, he said.
“The products have reached a point where they provide a lot of benefit to researchers,” McLaughlin explained, noting that in the past year or so he’s seen a shift in percentages: Previously most of Carestream’s molecular imaging customers were hard core imaging people; now, more customers are in application areas.
State-of-the-art optical molecular imaging systems enable noninvasive visualization of biological processes in vivo, enabling researchers to watch disease progression over time in the same animal. They use multiple fluorochromes to selectively target biological processes, and visualize small groups of cells (usually 50 is sufficient for research needs, though Oldfield says Caliper has followed tumors composed of just five cells–to demonstrate the technology’s capability). They enable testing at intervals to illustrate how tumors develop and respond to drugs, and their output can be co-registered with images produced by other modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) systems.
Moving up for drug discovery
For drug discovery, Oldfield says the technology has been used mainly at the end of the process, but is now being pushed much further upstream, to help determine which cell signaling pathways are affected by a drug. Previously the pathways were studied in vitro and millions of compounds were screened, he explains, but the newer approach lets researchers narrow down their work to perhaps 10 or 20 compounds, look at the pathways, learn what triggers this or that enzyme, and focus on compound optimization and drug efficacy. Oldfield says in vivo systems enable researchers to “fail faster” by getting the compounds into animals sooner so they can learn more quickly and accelerate the whole screening process. Observing disease progression in a live animal can provide all kinds of other information as well, he says.
Pharmaceutical companies don’t publish much (and are typically tight lipped about the technologies that help them get ahead), but Oldfield says he has just begun to see publications from the pharma labs demonstrating correlation between the upstream and downstream ends of the process.
In addition to this, in-vivo imaging is moving closer to clinical trials to enable testing of dosing levels. McLaughlin and Oldfield note that the approach has proven attractive for imaging of inflammation and for stem cell research. Explaining its use for imaging the inflammation that accompanies heart disease, McLaughlin explains that “vulnerable plaques have certain signatures of inflammation that indicate whether they are benign or active.” Oldfield points to observation of inflammation associated with asthma, arthritis, and stroke. A slideshow on Caliper’s website explains that all of the most commonly employed optical reporter labeling strategies have been used to generate light-producing stem cells; Oldfield explains that these can be seen tracking to the heart following cardiovascular damage.
The latest technology progress relates to 3D imaging for more precise pinpointing and quantification. Oldfield says Caliper has done much to improve software to enable this and make it easily accessible. And Carestream is working on a multimodal animal rotation system designed to eventually enable 3D visualization. The idea is to enable change of modalities (optical and x-ray) without moving the animal or focal plane–and register the imagery with precision. McLaughlin says the system will find the optimal angle for the optical signal and keep track of the rotation angle to enable tracking of changes over time.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.