IMAGING: World Molecular Imaging Congress highlights optics advantages

The title of the opening keynote address at the 2008 World Molecular Imaging Congress (Sept. 10–13; Nice, France), “Advances in Molecular Imaging from bench to bedside,” articulated one of three key themes of the meeting: translating preclinical work to clinical.
“The promise of molecular imaging is to provide an uninterrupted flow of technologies from in vitro microscopy and in vivo animal imaging to clinical phenotyping of disease and image guided therapeutic interventions,” said keynote speaker Dr. Markus Schwaiger of the Nuklearmedizinische Klinik und Poliklinik (Munich, Germany). Dr. Schwaiger discussed the advancement of molecular imaging, including probe development, which he said has become quite sophisticated as both a diagnostic tool and a method of therapy selection, “especially in the area of optical imaging,” he said. And indeed, probe development was another key theme of the conference.
Then Dr. Schwaiger went on to discuss a third major conference topic: multimodal imaging. “Optical imaging is now limited,” he said, but noted that situation is changing.
A new era of optical imaging
Dr. Vasilis Ntziachristos, director of the Institute of Biological and Medical Imaging (Munich, Germany) spoke of “a new era of optical imaging” during his presentation. Ntziachristos, named one of the world’s top innovators by MIT, said the number of published papers covering optical imaging has increased substantially recently, and added, “performance is coming together for optical.”
Michael Seiden, MD, PhD, of Fox Chase Medical Center (Philadelphia, PA), which has an equity position in VisEn Medical (Bedford, MA) and is helping to advance Phase I clinical trials of VisEn’s “smart” fluorescence-activatable imaging agents, gave one example of how this is happening: Optical systems can provide finer resolution than other techniques such as CT or PET, for instance highlighting tumors that are otherwise invisible.In discussing the fluorescence-assisted resection and exploration (FLARE) system for highlighting cancerous tissue during surgery, John Frangioni, MD, Ph.D., said that for cancer surgery, “optical is the only modality” that’s viable. Frangioni, of Beth Israel Deaconess Medical Center (Boston, MA) codirects its Center for Imaging Technology and Molecular Diagnostics. Now in early clinical trials, FLARE consists of a near-infrared (NIR) imager, a video monitor, and a computer.
Bill McLaughlin of Carestream Molecular Imaging (Rochester, NY) described an in vivo small-animal system in the wings that will enable multiview rotational imaging. Researchers will be able to dial in the exact angle of viewing and thus maintain consistency throughout lengthy experiments.
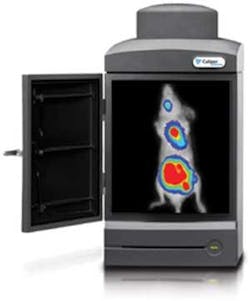
Out in the exhibit hall, Caliper Life Sciences (Hopkinton, MA) touted its new IVIS Kinetic real-time video imaging system, which leverages new EMCCD developments for noninvasive viewing of biological events at the molecular level as they occur.
Huge opportunity
In a presentation called, “From Cells to Sales: Commercial Opportunities and Challenges of Optical Molecular Imaging as a Clinical Tool,” Dr. Shahram Hejazi of AVA Biomed Partners (formerly president of Carestream Molecular Imaging) described the long and difficult process necessary for new therapeutics and devices succeed in patient care applications, including complex, expensive, and lengthy multiphase FDA trials; the similarly onerous process of securing approval for insurance reimbursements; and “the most difficult barrier,” market adoption. (And he echoed a sentiment heard elsewhere in the conference, saying that for a new piece of equipment to achieve market adoption, it must fit easily into the established workflow.)
He painted a much brighter picture, though, when he described the potential for optical molecular imaging as a pharmaceutical tool. He said that the clinical trial market, which is expected to grow from about $25 billion in 2007 to well over $30 billion in 2009, includes a $2 billion market just for imaging tools. “The opportunity to expedite drug development is huge,” he said.
Used for drug development, market adoption barriers are less significant, there is “no reimbursement challenge,” and the FDA’s less rigorous requirements apply. And he noted that molecular imaging is particularly useful and attractive in instances where the drug development cycle is very long. “Especially neurological diseases,” he said, “where change happens over years.”
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.