LASER SURGERY/CANCER TREATMENT: Real-time interactivity enhances interstitial brain tumor therapy
Laser interstitial thermal therapy (LITT) is a minimally invasive, low cost, and effective approach to neurosurgery. It is especially appealing when the surgeon can control its application for precision and accuracy.
By Richard Tyc and Kurt Jeff Wilson
Surgical resection and stereotactic radiosurgery (SRS) are the primary therapeutic interventions for the treatment of brain tumors, but they have drawbacks and limitations–and thus adjuvant therapies are considered critical for addressing brain cancer. Today's primary adjuvant therapies, radiotherapy and chemotherapy, can be devastating to patients, however, and options such as immunotherapy and gene therapy are just emerging. So an effective yet minimally invasive and low-cost option is particularly compelling for both clinicians and patients.
Controlled thermal ablation
All live human cells can be killed almost immediately at severely elevated temperatures (above 57°C) through various processes, including protein denaturation, coagulation, and vaporization. At lower temperatures (43°–57°C) tissular necrosis (tissue death due to protein denaturation) will occur with exposure from minutes to hours.1 Solid tumors in the breast, prostate, lung, liver, skin, and other organs respond to thermal therapy–the application of heat to destroy diseased tissue–in part because it can be applied minimally or noninvasively. Depending on the application and the technology, the heat source may be extracorporeal (outside the body), extrastitial (outside the tumor), or interstitial (inside the tumor).
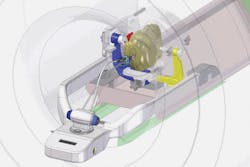
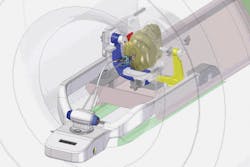
FIGURE 1. Within an MRI suite, a surgeon can operate the AutoLITT workstation to control the system's MRI-compatible laser therapy tools. Once the patient is positioned so that his/her head remains steady inside the MRI coil, a small opening is made in the patient's skull. The patient-probe interface (PPI) then establishes laser probe trajectory and provides stability during probe alignment, insertion, and operation.
Application of thermal therapy for treatment of brain tumors has rarely been an option. That's partly because of limited ability to deliver sufficient energy exclusively to and selectively destroy the tumor without damaging surrounding healthy brain tissue. It's also because of the limited ability to monitor temperature noninvasively with sufficient spatial and temperature accuracy and precision.
Laser interstitial thermal therapy (LITT) uses a low power (3–15 W) laser with a specialized probe to heat and coagulate a tumor from within. An advantage of LITT compared to externally applied energy (e.g., high-intensity focused ultrasound) is that the energy is applied directly to the tumor rather than passing through surrounding normal tissue. Also, energy deposition is more likely to extend throughout the entire tumor.
LITT was investigated for treatment of cerebral neoplasms in 1994 but at that point real-time imaging and temperature control were not applied. As has been documented in several case reports by a German research team, the risks of LITT therapy are low in the treatment of brain tumors–and benefits have been immediate for many. Reported improvements in survival following LITT therapy, although only available from small cohorts, are similar to those observed following surgical resection, with a median time of 11.2 months.2 (Though survival times in some cohorts were much greater than one year, this result could be evidence of selection bias.)
FIGURE 2. A set of real-time images, generated during the initial stages of a LITT procedure, shows how the surgeon confirmed probe alignment, starting with two different views of a patient's brain (upper and lower left). At right, the alignment/confirmation wand, filled with MR contrast, highlights the planned trajectory.
Most descriptions of thermal energy application have been based upon theoretical, a priori calculations of energy necessary to treat the specific tumor size, without real-time control of the energy delivery to match the actual tumor's geometry and heat conduction properties. Others have used MR images to treat in real time, but their technique shows only those cells that have immediately died during the treatment.3 This latter technique understates the treated tissue since it does not calculate the thermal damage experienced by the tissue beyond this immediately dead boundary.
Steerable for neurosurgery
The AutoLITT System, a LITT implementation by Monteris Medical (Winnipeg, MB, Canada), is a platform comprising MRI-compatible laser components that enable guided delivery of precision thermal therapy (see Fig. 1). The system received FDA 510(k) clearance in 2009 for use in neurosurgery. It promises another option to some brain tumor patients that may otherwise be unsuitable candidates for craniotomy or SRS. The system allows for conformal laser thermocoagulation of deep-seated brain tumor using a probe whose rotation and depth are surgeon-controlled, and it provides real-time quantitative temperature maps and thermal dosage zones (see Fig. 2), which predict therapeutic target tissue death.
Early clinical implementations of LITT for treatment of intracerebral tumors have used a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser and required irradiation times of up to 30 minutes for treatment of a single tumor.2, 4 In those cases, fiber-optic probes delivered the laser energy in a uniform distribution with respect to the fiber axis, resulting in a nominally ellipsoidal deposition of energy thermal treatment, and were used without real-time feedback control or prediction of thermal damage.
As a critical point of differentiation, AutoLITT technology uses a thin, side-firing probe (see Fig. 3). The design includes the laser optical fiber designed to emit at an angle to the probe axis, a gas-cooled sapphire tip, and a rigid structure for insertion via frameless stereotactic device. This design enables the thermal damage to be confined to a specific target tissue region. Rotation and translation of the probe allow the surgeon to direct the thermal treatment selectively, sculpting each section of the tumor, and to vary the treatment parameters according to the nature of the tissue in that target zone. In addition to playing a role in directional heating, the gas-cooled probe tip allows application of higher laser powers to create larger lesions (3–4 cm), speed lesion production, and increase safety.
The ability to monitor temperature and thermal dosage (which correlates to therapeutic benefit) in real time during MR imaging enables the surgeon to treat lesions closer to eloquent areas. Such interactive control of the thermal dosage needed to kill the tumor and monitor any potential damage to adjacent tissue, in conjunction with the ability to rapidly apply energy leading to sharper thermal gradients near the boundary of the thermal ablation zone is clinically beneficial.5
FIGURE 3. A surgeon-controlled side-firing diode laser probe provides directionality and targeting abilities for precise, accurate application of laser energy–and therefore can minimize unplanned thermal damage to healthy brain tissue.
The AutoLITT MRI image analysis software (VizApp) has been demonstrated in animal studies to successfully predict which tissue has received a thermal dose that will ultimately result in target tissue death. This, along with the directionality and targeting abilities of the side-firing laser probe, allows the physician to tailor the treatment of the lesion to the actual geometry and thermal conductivity properties of the tumor.
The central components of the system are:
- a gas-cooled laser probe that delivers controlled energy to a defined target tissue region;
- a probe driver that allows the surgeon to precisely position, stabilize, and manipulate the probe, endoscope, or other device within the target region;
- a patient-probe interface (PPI), a frameless stereotaxy device used to establish the probe (or other device) trajectory and stability during alignment, insertion, and operation; and
- VizApp software, including a user interface, for planning and monitoring treatment with the MRI and the hardware subsystems.
The AutoLITT components collectively interface with a 1064 nm wavelength diode laser (Dornier) to generate the energy used to heat tissue, and a Siemens 1.5T MRI system to provide image feedback for the positioning of the probe and to provide the data necessary for temperature feedback (thermometry) during treatment.
Since the AutoLITT probe is as small as many conventional biopsy needles and trocars (3.3 mm diameter, approx. 10F, or just under 10 Ga), it allows a minimally invasive approach to any target tissue, including the brain. When the probe is mechanically inserted through the PPI, the surgeon can use the probe driver to direct the thermal therapy to almost any region inside the skull through a small incision. The laser energy is transmitted from the remote diode laser, through connecting fiber optics, and then out the end of the probe to target the tissue. When embedded in the tumor, the probe tip is kept cool by allowing pressurized gas from a remote source to expand within the tip capsule, creating a Joule-Thomson effect. This gas is vented outside the body. Cooling the probe tip optimizes the thermal damage zone, allows for higher power density and prevents high temperatures near the probe that could cause unwanted charring, vaporization, or ablation.
FIGURE 4. This software output graphic, generated by VizApp, shows the minimum (outlined in yellow) and maximum (outlined in white) generated iso-dosage regions that guided the surgeon during laser therapy application. Positioning of the side-firing probe is indicated in turquoise, while the center arrow depicts the direction of the laser energy.
The VizApp software converts MRI time-temperature data to display an algorithmic computation of thermal dosage. This assists the surgeon in monitoring and controlling the delivery of the thermal therapy. The software also provides treatment planning, trajectory registration, hardware interfaces, and important safety interlocks.
Data processing and real-time presentation
A major advance in LITT is the ability to monitor more than just the current temperature of the MRI visualized tissue, and to determine the "thermal dose" to which the tissue has been exposed. Thermal dose is a means of measuring a treatment that is clinically relevant to a biological effect. Sapareto and Dewey developed the standard method used today, which calculates the time and temperature history of a tissue and translates the result as an exposure time at some reference temperature.1 The reference temperature commonly used in reporting experimental thermal exposures is 43° C.6 Based on literature results and confirmed by Monteris animal studies, the longer a tissue is at a temperature greater than 43°C or the higher the temperature above 43°C, then the more likely the tissue will die.
VizApp evaluates the instantaneous temperature and the time-temperature history to produce a thermal dose profile across three MRI image slices perpendicular to the probe axis. Except for enabling emergency shutoffs, the software at no time controls the laser energy; instead, the physician controls the positioning of the laser probe and the application of the laser energy at all times.
In clinical investigations, once the laser had been activated inside the target tissue, the software first displayed a yellow thermal iso-dose line on the MRI image (see Fig. 4). All cells within the yellow iso-dose line had been exposed to the thermal dosage demonstrated to have a detectable effect–which, depending on many factors, could range from a nonphysiological effect (most likely) to cell death (very unlikely). All tissue outside of this yellow boundary line had been demonstrated in animal studies to experience no irreversible changes or damage. As the laser energy continued to be delivered, a blue iso-dose line appeared. The blue boundary indicates tissue that had been exposed to the thermal equivalent that was expected, again based on prior results, to cause significant damage and necrosis within two days, depending on multiple physiological factors. The final thermal iso-dose line to emerge was white, indicating the boundary of tissue that had been exposed to the thermal dosage previously shown to cause near-immediate tissue death. Typical laser energy applications were <5 min, depending on the size and geometry of the tumor. Multiple applications of laser energy were necessary at different angles and levels. Following a LITT treatment, the treated target mass eventually shrinks in volume, presumably as tissue is resorbed through natural processes.
The current commercial version of the AutoLITT system has incorporated the results of such studies and allows for the surgeon to interactively visualize the delivery of a therapeutic dose and the margins of that dose.
Now and in the future
As with all surgical procedures, AutoLITT is limited to the margins of the treatment area defined by the surgeon. For highly infiltrative tumors, such as glioblastoma multiforme (GBM), surgical interventions will not eliminate tumor cells that are diffused through the brain separate from the tumor mass itself. However, gaining local control over the tumor mass is generally the first objective of treatment.7
A recently completed clinical study (manuscript in preparation) demonstrated that AutoLITT treatments are a safe means of ablating recurrent GBM tumors to achieve local control, and represents another clinically useful therapeutic option for patients with intracerebral tumors. The minimally invasive nature and interactive surgeon control of the procedure, coupled with the advantage of real-time MRI monitoring, suggests utility in brain tumors where margins are best determined by MRI. It may also be useful to treat older patients in reduced physical condition, and to treat otherwise inaccessible regions of the brain. Furthermore, the nonionizing nature of laser energy may be beneficial in specific cases for which the ionizing energy used in radiation therapy and radiosurgery poses unacceptable risks.
This therapy is a prelude to the future development of minimally invasive procedures using interventional MRI techniques in neurosurgery. Continued analyses of thermal damage correlations with thermal dosage delivery and trials in other clinical settings are warranted.
The authors would like to acknowledge the Cleveland Clinic and University Hospitals Case Medical Center (Drs. Gene Barnett and Andrew Sloan) for clinical execution, data, and results; and Dr. Mark Torchia, Health Science Center, Winnipeg, MB, Canada, for technical guidance and manuscript review.
References
- S.A. Sapareto and W.C. Dewey, Int. J. Radiat. Oncol. Biol. Phys. 10, 6, 787–800, 1984.
- E. Briceño et al., Surg. Neurol. 67, 4, 388–91, 2007.
- Y. Anzai et al., AJNR Am. J. Neuroradiol. 16, 1, 39–48, 1995.
- T. Kahn et al., J. Comput. Assist. Tomogr. 18, 4, 519–32, 1994.
- T.J. Vogl et al., Radiology 209, 2, 381–5, 1998.
- R.J. McNichols, Int. J. Hyperthermia. 20, 1, 45–56, 2004.
- P.C. Schulze et al., Acta Neurochir (Wien). 146, 8, 803–12, 2004.
Richard Tyc is vice president of technology and advanced development; and KURT JEFF WILSON, Ph.D., is vice president of clinical and regulatory affairs, for Monteris Medical, Winnipeg, MB, Canada; e-mail [email protected]; www.monteris.com.
More Brand Name Current Issue Articles
More Brand Name Archives Issue Articles