Tool harnesses optogenetics to study protein condensation in living cells
By using optogenetics (which involves proteins whose behavior can be altered by exposure to light), researchers at Princeton University (New Jersey) have begun to explain how proteins assemble into different liquid and gel-like solid states—a key to understanding many critical cellular processes.
Related: New optogenetics switch could increase understanding of cell signaling
The researchers harnessed optogenetics by developing a new tool, dubbed optoDroplet, that offers unprecedented access to manipulating and understanding the chemistry that allows membraneless organelles to function. The tool, explains Clifford Brangwynne, an assistant professor of chemical and biological engineering at Princeton and senior author of a paper describing the work, will someday allow them to better understand the rules of physics and chemistry that govern the self-assembly of membraneless organelles. It could also lead to developing interventions and treatments for devastating diseases connected with protein aggregation, he says, such as amyotrophic lateral sclerosis (ALS; a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord).
Previous research has demonstrated that membraneless organelles assemble within the cell by a process known as a phase transition: examples of familiar phase transitions include water vapor condensing into dew droplets or liquid water freezing into solid ice. Studies over the last several years by Brangwynne and colleagues have revealed that altering the concentration of certain proteins, or modifying their structure, appears to trigger a phase change that allows proteins to condense into droplet-like organelles.
To date, though, most studies have used purified proteins studied in test tubes, and researchers have had few methods to study phase transitions in living cells. The OptoDroplet tool will help scientists learn about when phase transitions go awry, yielding solid-like gels and crystalline aggregates of proteins implicated in diseases such as Alzheimer's and ALS.
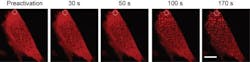
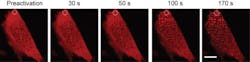
With OptoDroplet, the researchers showed that they could induce phase transitions and create membraneless organelles by switching on the light-activated proteins. They also could undo the transitions by simply turning the light off. Increasing the light intensity and protein concentrations allowed the researchers to further control the transition. By changing those inputs, they can determine when condensed liquid protein droplets form, as well as solid-like, protein aggregates, possibly linked to diseases.
Using mouse and human cells, the research team spliced in a gene for a light-sensitive protein from a plant called a mouse-ear cress (or Arabidopsis thaliana), a relative of cabbage and mustard that is a mainstay of genetics research. Blue light exposure causes the protein to self-associate, scrunching up on itself. The light-sensitive tag was fused to protein components thought to drive phase transitions in living cells. Using the light, the researchers found that they could induce the proteins to huddle up, mimicking the condensation process that naturally occurs in cells.
The research team repeatedly prompted the proteins to condense and then dissolve by turning the light on and off. The process proved fully reversible, even after many cycles. However, with high-intensity light or high concentrations of proteins, the researchers created semi-solid gels. Those gels were initially reversible, but over time they solidified to form irreversible lumpy aggregates, similar to those found in some diseases.
For example, the FUS protein—which is critical for a cell's operations—helps produce other proteins and repair damaged DNA. But scores of genetic mutations can cause the FUS protein to become too sticky, leading to ALS. The disease is marked by clumps of protein accumulating in nerve cells, and those clumps might stem from FUS or other proteins pathologically aggregating instead of staying as dynamic fluid droplets. Huntington's disease and Alzheimer's also involve clumps of proteins clogging up cells, again suggesting that abnormal phase transitions in cells are closely connected with these conditions.
Edward Lemke, a researcher at the European Molecular Biology Laboratory (Heidelberg, Germany) who was not involved in the work, noted the promise of optoDroplet: "The proteins targeted by optoDroplet are an important constituent of phase-separating proteins, many of which are also associated with infamous diseases. The optoDroplet system gives access to modulating the state of these proteins inside the cell in a minimally invasive and highly controlled fashion, so it can provide new insights on how they carry out their function."
Brangwynne and colleagues look forward to continuing to experiment with optoDroplet to better understand cells' complex behaviors. They are looking to also understand how cells can become diseased and, eventually, cured.
Full details of the work appear in the journal Cell; for more information, please visit http://dx.doi.org/10.1016/j.cell.2016.11.054.