Optical tweezers enable study of protein droplets that may play role in disease
Physicists at the University at Buffalo (UB; Buffalo, NY) are studying the properties of proteins that cluster together to form spherical droplets inside human cells. The research sheds light on the conditions that drive such droplets to switch from a fluid, liquidy state to a harder, gel-like state, which could help researchers better understand, for example, neurodegenerative diseases.
The study finds that certain protein droplets harden, becoming gelatinous in crowded environments such as test tubes where lots of other molecules are present, mimicking the congested conditions inside living cells.
"These droplet-forming proteins are a relatively new area of study, so we know very little about their basic properties," says lead investigator Priya R. Banerjee, Ph.D., assistant professor of physics in the UB College of Arts and Sciences. "As physicists, we want to quantify the dynamics of these droplets and learn what factors influence them. This is important as the dynamics of protein droplets are a key to their cellular function and dysfunction."
Banerjee adds that while prior research has focused on the structure of the proteins themselves, his team's work shows that environmental factors are equally important. "We see that external conditions can alter the internal state of the droplets, which may affect their function in human cells," he says.
Condensating proteins may be involved in health and disease, as recent studies point to potential roles for these droplets in functions such as gene expression, stress response, and immune system function.
In the research team's paper that describes the work, they investigate a droplet-forming protein called fused in sarcoma (FUS). Liquid FUS droplets are found in normal brain cells, but in some patients with the neurodegenerative disease amyotrophic lateral sclerosis (ALS; also known as Lou Gehrig's disease), the protein forms aggregates of solid material, Banerjee says.
Related: Fluorescence technique identifies misfolding proteins that cause diseases
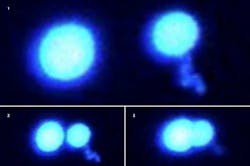
In their first set of experiments, the researchers used highly focused laser beams known as optical tweezers to trap and push together two protein droplets floating in a liquid buffer solution. The protein droplets merged easily to form a single larger droplet when the buffer was thinly populated with other inert crowder molecules such as polyethylene glycol (PEG). But when the concentration of PEG or other chemicals in the buffer increased, the protein droplets became more gelatinous and would not fully combine.
In a second set of tests, the team employed lasers in a different way—"laser poking"—to study how FUS and related protein droplets react to crowded environments. In these experiments, they attached fluorescent tags to numerous protein molecules in a single droplet, causing the proteins to glow. The researchers then "poked" the middle of the droplet with a high-intensity laser, a procedure that caused any fluorescent molecules hit by the laser to go permanently dark.
Next, scientists measured how long it took for new glowing proteins to move into the darkened area. This happened quickly in protein droplets floating in sparsely populated buffer solutions. But the recovery time was dramatically slower for droplets suspended in buffer solutions thick with PEG or other compounds, which indicated that protein droplets become gelatinous in crowded environments. The findings applied to both FUS and other related protein droplets with diverse primary structures.
"Our experiments were done in test tubes, but our results suggest that inside living cells, the crowding status could affect the dynamics of protein droplets," Banerjee says.
One important question that remains is whether and how the fluidity of FUS droplets impacts the protein's ability to form into solid clumps, as seen in some ALS patients. Banerjee hopes to address this problem through future research.
Full details of the work appear in the journal Biomolecules.