Light-activated polymer shows promise in surgical guidance, plus kills malignant cells
Researchers at Oregon State University (OSU; Corvallis, OR) have developed a new way to selectively insert compounds into cancer cells—a system that will help surgeons identify malignant tissues and then, in combination with phototherapy, kill any remaining cancer cells after a tumor is removed.
Related: 'Painting' tumors to guide cancer surgery
The findings have shown remarkable success in laboratory animals. The concept should allow more accurate surgical removal of solid tumors while also eradicating any remaining cancer cells. In laboratory tests, it completely prevented cancer recurrence after phototherapy.
Technology such as this, the scientists say, may have a promising future in the identification and surgical removal of malignant tumors, as well as using near-infrared (NIR) light therapies that can kill remaining cancer cells, both by mild heating of them and generating reactive oxygen species that can also kill them.
"With this approach, cancerous cells and tumors will literally glow and fluoresce when exposed to near-infrared light, giving the surgeon a precise guide about what to remove," explains Oleh Taratula, an assistant professor in the OSU College of Pharmacy. "That same light will activate compounds in the cancer cells that will kill any malignant cells that remain. It's an exciting new approach to help surgery succeed."
The work is based on the use of a known compound called naphthalocyanine, which has some unusual properties when exposed to NIR light. It can make a cell glow as a guide to surgeons, heat the cell to kill it, and produce reactive oxygen species that can also kill it. And by adjusting the intensity of the light, the action of the compound can be controlled and optimized to kill just the tumor and cancer cells. This research was done with ovarian cancer cells.
However, naphthalocyanine isn't water-soluble and also tends to clump up, or aggregate, inside the body, in the process losing its ability to makes cells glow and generate reactive oxygen species. This also makes it difficult or impossible to find its way through the circulatory system and take up residence only in cancer cells.
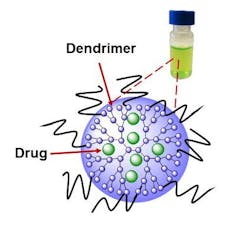
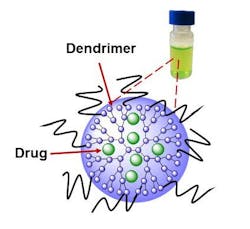
The OSU research team overcame these problems by use of a special water-soluble polymer, called a dendrimer, which allows the napthalocyanine to hide within a molecule that will attach specifically to cancer cells, and not healthy tissue. The dendrimer, an extremely tiny nanoparticle, takes advantage of certain physical characteristics that blood vessels leading to cancer cells have, but healthy ones do not. It will slip easily into a tumor, but largely spare any healthy tissue.
Once in place, and exposed to the type of light needed, the cancer cells then will glow—creating a biological road map for a surgeon to follow in identifying what tissues to remove and what to leave. At the same time, a few minutes of this light exposure activate the naphthalocyanine to kill any remaining cells.
Before attempting human clinical tests, the OSU researchers hope to perfect the process and then collaborate with Shay Bracha, an assistant professor in the OSU College of Veterinary Medicine, to test it on live dogs that have malignant tumors. The technique has already been shown successful in laboratory mice. Worth noting, the researchers say, is that even as phototherapy was destroying their malignant tumors, the mice showed no apparent side effects and the animals lost no weight.
Systems with technology similar to this are also being tested by other researchers, but some of them require several imaging and therapeutic agents, repeated irradiation, and two lasers. This increases cost, may lessen effectiveness, and increase risk of side effects, the OSU researchers say.
Full details of the work appear in the journal Nanoscale; for more information, please visit http://dx.doi.org/10.1039/C4NR06050D.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!