U.S. FDA clears LaserOptek picosecond laser for use in dermatology, surgery
Aesthetic and medical laser maker LaserOptek (Seoul, South Korea) has received U.S. FDA clearance for its PicoLO picosecond Nd:YAG laser, indicated for use in dermatology and general and plastic surgery.
The laser generates high peak power and consistent picosecond pulse duration, producing strong photomechanical forces within the skin to quickly and effectively break down and fragment pigments such as those found in tattoos. The laser incorporates the company's Diffractive Optical Element (DOE) fractional technology to create Laser-induced Optical Breakdown (LIOB) in the dermis, even at low power.
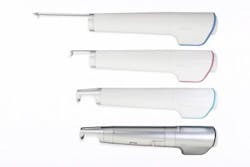
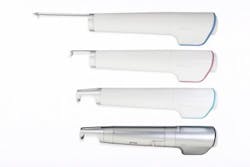
Laseroptek's PicoLO hand pieces (zoom, fractional, collimated, 585 nm dye, and 650 nm dye) are shown.
This new approval comes shortly after the company received U.S. FDA clearance for its LOTUS III multipulsed Er:YAG laser at the end of 2018. Both systems are well received in multiple international markets and the company is now in the process of establishing an exclusive, full-service dealer network in North America, according to C.J. Lee, CEO of LaserOptek.
For more information, please visit laseroptek.com.