BIOMEDICAL IMAGING/DISEASE DIAGNOSIS: Quality and standards: Making bioimaging "measure up"
Today's imaging tools provide unprecedented views of biological processes—but they fail to quantify the exact location and extent of disease or illustrate how disease states change over time. What's more, imaging studies vary by system and geographic location, so that results from a study done in California may not match up with results from the same type of study done in New York. And image interpretation—either by computer or by a human—introduces variability that can skew results.
Whether developing a new drug or imaging agent, or working to diagnose disease, quantitative results—comparable across time and space—are crucial. To move imaging techniques closer to quantitative assessment tools, a number of organizations—including the National Institute of Standards and Technology (NIST), the U.S. Food and Drug Administration (FDA), the Radiological Society of North America (RSNA), system manufacturers, and academic partners—have convened working groups. The aim of these partnerships is the creation of a range of imaging parameters traceable to standard references.
As part of these efforts, NIST will address standards needs in laboratory medicine and medical imaging. The approach is three-pronged:
- develop standards for increased quality in current generation biomedical measurements for medical imaging and in vitro diagnostics and therapeutics,
- create standards to support next-generation healthcare measurements in human cells, fluids, and tissues such as DNA, RNA, protein and metabolite measurements, and
- produce standards to support manufacturing and regulatory approval of biologic drugs.
"We are providing measurement and standards tools to allow medical imaging and laboratory testing to move from an art to a science," says Willie May, director of the Chemistry Science and Technology Laboratory at NIST. "To predict and prevent [disease] you need to be able to measure."
Creating national standards for imaging systems and devices is just one activity in a complex matrix that supports commercial biomedical markets. The standards matrix includes NIST, the FDA, Congress, and industry and professional groups. Each organization plays a role in creating a system that can ensure systems and devices used in both laboratory and clinical science are as accurate as possible. NIST evaluates the science and creates the traceable references. FDA's device approval process, the gateway to commercial markets in the U.S., asks manufacturers to prove their systems are reliable. Congress supplies the financial support for standards research and industry and professional groups help evaluate what standards are needed and promote implementation.
Although no formal timeline exists for developing standards for imaging systems, it may take a decade before the full impact of imaging standards hits the research and clinical communities. But make no mistake: The process has begun. Those involved say that in addition to creating the standards, a parallel education process needs to occur to help system developers and users understand the new standards. System manufacturers will need to agree to follow the standards and that implementation could be costly in the short-run.
"Small and large companies have to pick and choose how they will spend their time, money, and effort," says Randy Prebula, director of regulatory sciences at Hogan and Hartson, Washington, DC. "Some may have to weigh the tradeoffs but standards will be part of the equation." However, "the pay-off [of standards development] in the long-run is serving the interests of patients and physicians," explains David Armbruster, scientific affairs manager at Abbott Laboratories.
FDA's role
Historically, FDA has not required companies to follow standards in order to gain device or drug approval. Companies describe their potential product, show why it's reliable, and explain why it's better than products currently on the market. "There may be a new device that is better than the ones currently in use, but FDA could refuse to approve the new device because it doesn't match existing products," says Armbruster. Approval hasn't hinged on proving that a product is traceable to a standard reference.
But FDA experienced a great deal of change in 2009. A new FDA commissioner was installed as well as a new director of FDA's Office of In Vitro Diagnostic Device Evaluation and Safety (OIVD) while the long-time head of the Center for Devices and Radiological Health resigned. With new people in key positions, it's likely that FDA's drug and device approval process will come under scrutiny. Developers interested in getting imaging agents, diagnostic tests, or medical devices approved should ensure that their quality control procedures are well-documented.
Those who track medical device approvals suggest that companies collect strong data that supports a device's claims and intended use. New imaging technologies will likely be subject to the new standards, but it's unclear how FDA will enforce adherence to those standards and whether the agency will require existing imaging devices to be reevaluated based on the new standards. "We can't predict what will happen, but we can hope that the agency and industry will police themselves," says Susan Tiedy-Stevenson, senior director of regulatory sciences at Hogan and Hartson.
Congress's role
NIST is enthusiastically adapting its measurement expertise to the life and medical sciences. A new intramural research program, Optical Imaging for Clinical Applications, just received internal NIST funding of $1.1 million for the next five years. Industry also sees a need to make better use of current and emerging imaging techniques. Congress, however, appears lukewarm when it comes to funding.Since FY2007 Congress has provided NIST with $4 million to develop medical imaging standards. NIST requested an additional $3.55 million for the effort in FY2010, but as of press time, Congress had not approved NIST's 2010 budget and the agency was operating under a continuing resolution. It's unclear how willing Congress is to support standards work. The House Subcommittee on Technology and Innovation, which oversees NIST, held a hearing in September 2009 on the need for standards in biologic drug development and, at the time, this hearing was billed as the first of a series of hearings on standards in healthcare. Sources on Capitol Hill suggest that the committee is unlikely to follow up with additional hearings any time soon.
Reducing costs
Devising references traceable to a national standard will yield quantitative results for imaging techniques, which will translate into better science as well as healthcare cost savings. A 2005 Medicare Payment Advisory Commission (MedPAC) report to the U.S. House of Representatives Subcommittee on Health, Committee on Ways and Means found that diagnostic imaging was a major source of healthcare costs (see medpac.gov/publications/congressional_testimony/031705_TestimonyImaging-Hou.pdf).
In addition to creating more meaningful study results across locations and imaging systems, imaging standards could also lower cost by reducing the size and time requirements of clinical trials. Measuring a chemical endpoint rather than waiting for a clinical outcome would streamline the drug and device development process. Currently clinical trials enroll hundreds or thousands of patients at multiple locations. "You end up proving a technology or therapy with statistics," says William Ott, deputy director of NIST's Physics Laboratory. With increased imaging precision, trials in the future could demonstrate a drug or treatment's effectiveness in days rather than weeks.
To move toward this model, RSNA established the Quantitative Imaging Biomarkers Alliance (QIBA) in 2008. The group's focus is on "building 'measuring devices' rather than 'imaging devices.'" Three technical committees, FDG-PET/CT, dynamic contrast enhanced MRI, and volumetric C, are identifying sources of variability within each technology, creating baseline data and developing profiles–guidelines that describe what will be achieved by following the profile as well as what manufacturers and users must do to achieve the stated outcome.
Optical references
Unique challenges face in vivo optical measurements—the presence of water on the surface, changeable viewing or detection geometry, sometimes the necessity of changing spatial scales, and sample motion. Currently, no measurement standards for optical imaging exist that are traceable to national standards for radiometric or spectrophotometric quantities such as color, appearance, brightness, reflectance, and transmission in human tissue.
To address this challenge, NIST's Optical Imaging for Clinical Applications research program is developing a database of optical properties for tissue using data gathered in actual surgery. The database will provide a library of different tissues, from brain to skin, along with their associated spectral information. In addition to leading efforts to create tissue phantoms—models that simulate human organs both healthy and diseased—for CT, MRI, PET, and PET-MRI, NIST is working with several academic institutions to develop a digital tissue phantom as a calibration tool. The tool is designed to re-project images complete with spectral content.In the case of surgical imaging devices, spectral reflectance standards used to determine color in a number of manufacturing industries are being adapted for use with human tissue. By calibrating a surgical imaging tool to the spectral standard, surgeons can be confident that the colors they see through their instrument are true.
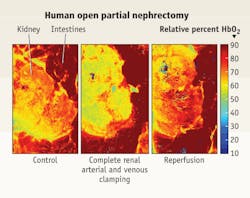
"Currently surgeons make a relative measurement based on tissue color, draw a line and say this side is cancer, this side is not," says Ott. The healthy tissue may be pink and the diseased tissue white. Because color vision is highly individualized, NIST is also collaborating with researchers at the University of Texas, Arlington to create an imaging system that can measure the reflectance properties of both normal and diseased tissue.
A challenge facing optical imaging for diagnosis and treatment is the material under observation. "Optical technology has always been used in the lab as an analytical technique," explains Maritoni Litorja, a researcher in the Optical Technology Division of NIST's Physics Laboratory. "The specimens are pure and the instruments calibrated based on those specimens. It's a big jump from pure compounds to living tissue. With tissue you're at the mercy of what the sample gives you. It gets very murky." (For information on references development for non-optical methods, see the sidebar, "RE: Non-optical approaches.")
Early returns
The early efforts by NIST and industry working groups are starting to pay off. Phantoms under development will enable calibration of imaging systems to a standard reference. If imaging centers calibrate their systems to the same phantom, the results are likely to be comparable across centers. Comparable results across sites are particularly important in clinical trials. The standards collaborations are also creating reference libraries of spectroscopic tissue data and more consistent patient dosing for imaging tracers.
Developing standards for imaging techniques will be a process—one that involves consensus and partnership. The exquisite pictures rendered from well-designed imagers calibrated to a national reference will not only show key details of normal and disease states but will offer researchers and clinicians reliable and comparable results. Standards promise to move imaging from the interpretive realm of art to the quantitative precision of science.
RE: Non-optical approaches
Optical methods are part of a larger palette of biomedical imaging options that include—and are increasingly integrated with—more established modalities. So understanding the standards efforts underway in those areas can put things in context for optical imaging specialists.
CT references
An estimated 100 million computed tomography (CT) scans are performed annually around the world, according to Kaiser Permanente—yet CT's power to provide volume information about tumors remains unrealized. Improvements in CT's ability to detect volume changes in tumors could lead to more widespread use of the modality for early cancer detection.
Early detection of cancer increases survival rates for patients and is particularly critical for those affected by lung cancer. In the US alone, says the American Cancer Society, 200,000 new cases of lung cancer are diagnosed each year and 160,000 people die annually. Research has shown that CT screening of high-risk groups could mean a survival rate as high as 80%. Because CT image analysis software does not provide quantifiable and reproducible tumor measurements, few clinicians choose the x-ray based approach as a diagnostic tool for lung cancer. To improve CT measurements, NIST is developing phantoms and standard protocols, creating methods to characterize tumors with known certainties, and evaluating reconstruction and analysis algorithms.
CT calibration standards could also improve control of radiation exposure. During the past year a debate has slowly simmered in the radiology community about patient exposure to too much radiation during CT scans. In a number of cases, patients received excess amounts of radiation. Tighter exposure tolerances could eliminate similar missteps.
MRI references
In the case of magnetic resonance imaging (MRI), NIST working in conjunction with the International Society for Magnetic Resonance in Medicine has developed the first MRI phantom. The model will determine the geometric accuracy of MRI scanners, resolution, signal-to-noise levels, and system stability. NIST is also developing measurement systems to characterize MRI nano-contrast imaging agents including measuring activation of individual nanoagents and detecting nuclear MR relaxivity around a single nanoagent.
PET references
Positron emission tomography (PET) reveals chemical and metabolic processes in the human body. Its power lies in its ability to examine metabolic activity in tumors, track cardiac function, and demonstrate which parts of the brain react during various activities. Calibrating PET scanners is challenging because the radioactive isotope, fluorine-18, used in many PET studies only lasts a short time before it's signal goes dark. Without a reference for fluorine-18, PET measurements taken at multiple centers, in multiple patients, and in the same patient over time cannot be compared.
To overcome this difficulty, NIST researchers and RadQual (Weare, NH), a supplier of quality control products for nuclear and PET imaging, developed a calibration surrogate for fluorine-18 that became available in 2009. The standard uses germanium-68 embedded in an epoxy to simulate fluorine-18 in a syringe. This helps researchers more accurately determine the amount of fluorine-18 to inject into patients during the PET scan so as to minimize radiation dose while maximizing image resolution.
In a multicenter trial using the NIST traceable germanium-68 reference, 39 calibrators were evaluated. Using the manufacturer's preset values for germanium-68 and fluorine-18 the calibrators overestimated the actual activity in the syringe by an average of 7%.
Combining methods
To address the growing interest in combining CT with PET and MRI, NIST will soon begin standards work for PET-MRI and PET-CT. Currently no phantoms are available to calibrate PET-MRI systems. Once developed, the phantoms will help to quantify images in terms of spatial dimension and contrast/positron emission intensity.
On the PET-CT front, NIST plans to obtain a PET-CT imager using stimulus recovery funds. The scanner will be calibrated for absolute activity and serve as a national reference for phantom characterization and calibration, a testbed for imaging physics studies, and an opportunity to study factors influencing image quantification. The datasets captured from the new scanner will also serve as reference data for software validation.
About the Author