OPHTHALMOLOGY/MICROCAMERA TECHNOLOGY: FDA approves artificial retina
The first-ever artificial retina implant, designed to replace damaged cells within the eye, has received market approval from the U.S. Food and Drug Administration (FDA). Second Sight Medical Products (Sylmar, CA) commercialized the Argus II Retinal Prosthesis System after nearly two decades of development involving scientists and engineers from academe, government, and industry supported by private and public investment, including funding from the National Institutes of Health, the National Science Foundation, and the U.S. Department of Energy.
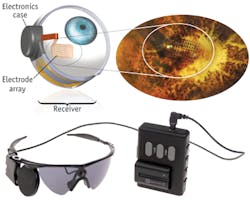
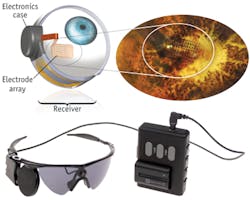
The Argus II includes a miniature video camera mounted on specialty eyeglasses, which streams images to a video processing unit (VPU) that the patient wears on a belt. The VPU processes the video, transforms it into instructions, and sends the data back to the glasses through a cable. These instructions are transmitted wirelessly to an antenna in the implant. The signals are then sent to the electrode array, which emits small pulses of electricity that bypass the damaged photoreceptors and stimulate the retina's remaining cells. The cells then transmit the visual information along the optic nerve to the brain. This process creates the perception of light patterns, which patients can learn to interpret as visual patterns.
The FDA approval applies to people affected by severe to profound retinitis pigmentosa (RP), a genetic disorder for which there was previously no effective treatment. The disease impacts one in every 4,000 Americans; the implant enables some affected patients, who are completely blind, to discern shapes such as large letters, and to locate objects, detect movement, and improve orientation and mobility.
The external hardware and software is upgradable—that is, designed to take advantage of future technology developments. And the system's video processor has settings that allow the wearer to adjust edge detection and contrast, for example.
The Argus II is slated to become available later this year in clinical centers across the U.S. Second Sight is looking to add sites to make the therapy more readily available.
A study of 30 patients with the condition showed that most were able to perform daily activities better with the implant than without it. Activities included navigating sidewalks and curbs, matching different color socks, and recognizing large words or sentences.
While the Argus II represents a breakthrough, the researchers hope to improve it further. Second Sight hopes to eventually win approval to treat other vision disorders.