Ultrafast laser light induces damage to DNA, with good implications for radiotherapy
Scientists at the Tata Institute of Fundamental Research (Mumbai, India) have shown that damage to DNA can be induced by high-intensity ultrafast laser light. The findings have important implications in clinical conditions, especially in reducing collateral damage to tissues surrounding the real target of conventional radiotherapy.
Related: Ultrafast laser enables cell study, with big implications
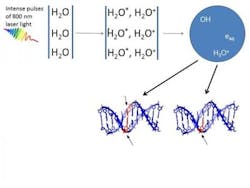
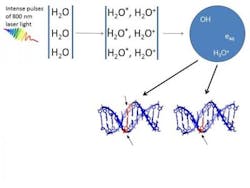
High-intensity femtosecond laser pulses were used to probe damage to aqueous DNA. In propagating through the water medium, the intense light pulses cause H2O molecules to ionize and break up, giving rise to low-energy electrons and OH-radicals. Both are responsible for producing breaks in DNA strands. Earlier work carried out by the researchers showed that OH radicals were four times more likely than electrons to produce double-strand breaks in DNA.
A collaborative project between TIFR Mumbai, the Centre for Excellence in Basic Sciences, Mumbai, and Manipal University, the experiments used different incident laser energies and various external focusing conditions to establish that DNA damage occurs in two distinct regimes. Interestingly, the numerical aperture of the focusing lens (the light-gathering ability of the lens) delineates the two regimes. This permits optical control to be exercised over the extent of DNA damage by simply varying the focal length of the focusing lens.
"The experimental technique of generating, in situ, slow electrons and radicals within aqueous media has important implications in different scenarios where the effects of nonionizing radiation need to be probed under physiologically relevant conditions," says Professor Deepak Mathur, senior scientist at TIFR Mumbai and the lead scientist of the study.
It has been suggested that detrimental dose distributions within tissues that are irradiated by gamma radiation—one of the major difficulties in radiotherapy—might be avoided by use of femtosecond laser-induced filamentation. This is because of ultrashort laser pulses, particularly in the infrared region, being spatially confined to volumes (~125 µm3) that are much smaller than what is possible to attain using contemporary clinical radiation sources. This is important for minimising damage to nontarget tissues in the vicinity.
Full details of the work appear in the journal Scientific Reports; for more information, please visit http://dx.doi.org/10.1038/srep27515.