OPTOFLUIDICS: Optofluidics enhances cytometry
Changhuei Yang, David Erickson, and Demetri Psaltis
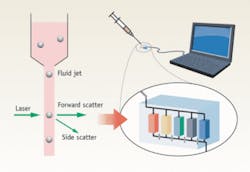
Flow cytometry is a mature clinical diagnostic technique that combines fluid flow and optical measurements to distinguish and determine the proportion of different cell types in blood. The general scheme involves flowing the processed blood in a very narrow and fast jet across a laser beam or a light beam. By detecting the amount of forward- and side-scattered light, a determination of the cell’s type can be made (see Fig. 1). Commercial flow cytometry machines are widely used. Chances are that, when you have your blood drawn for diagnostic purposes, your blood would be analyzed by a flow cytometer that is situated within a hundred feet of your blood draw location.
Clinicians use flow cytometry to diagnose diseases and track treatment prognosis. For example, a parasitic infection and a bacterial infection each trigger the increase of different white blood cell types. In recent years, cytometry has also been used to identify and count circulating tumor cells in blood. These are cells that have dissociated from a cancer tumor into blood circulation and that spread cancer beyond the primary tumor. Unfortunately, because the proportion of tumor cells in blood is extremely low—there is typically only one tumor cell in a milliliter of blood—cost-effective technology for identifying them with sufficient sensitivity remains elusive.
In recent years, several research groups and commercial endeavors have begun to translate flow cytometry into a microfluidic platform. In its simplest format, the approach replaces the conventional jet flow with flow in a microfluidic channel that is sufficiently narrow so that each cell can be interrogated individually. There are compelling reasons driving this transition; compactness and cost are two of the major ones. It’s easy to envision multiple compact, low-cost microfluidic-based cytometry units running in parallel to dramatically increase throughput.
The transition of flow cytometry from a free-space jet-flow format to a microfluidic system platform opens up unique opportunities for optical innovations, which can be easily incorporated onto the same platform. These optical improvements can improve flow cytometry in two major directions. First, they can dramatically improve sensitivity and bring in new sensing capabilities. Second, they can introduce new means for cell manipulation and sorting.
In fact, enhancing the capabilities of flow cytometry is a particularly appropriate application area for the nascent research field of optofluidics—the combination of optics and microfluidics to implement chip-level systems.1
Lensless high-resolution on-chip microscopy
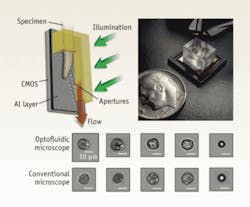
Flow cytometry has traditionally relied on nonimaging techniques for cell identification, so it is intrinsically less accurate than microscopy analysis. The optofluidic microscope offers an imaging solution that is particularly appropriate for microfluidics-based flow-cytometry systems (see Fig. 2).2 This microscope provides a simple, lensless, and high-resolution microscopy imaging method that is easy to implement in semiconductor fabrication facilities. In many ways, the optofluidic microscope represents a complete redesign of the conventional microscope so that a microscope can be shrunk onto a semiconductor chip.The optofluidic microscope consists of a line of small apertures spanning the metal-coated floor of a microfluidic channel. The device is illuminated from the top and the light transmission through each aperture is collected by a sensor pixel directly beneath the channel floor. As an object passes across each aperture, the measured time-varying transmission represents a line scan across the object. Therefore, by compiling the line scans associated with all the apertures, a high-resolution microscope image of the object can be obtained. The resolution is approximately equal to the aperture size, which can be as small as 500 nm.
This low-cost and compact microscopy approach is an excellent fit with flow cytometry, as it can be simply implemented and it provides much-needed image information.
Examining cells’ biochemical content
Beyond looking at the general cell morphology, it is also very useful for the clinician to be able to peek into the biochemical contents of certain cells. Fluorescence-based flow cytometry can already address some of these needs, but it has key limitations. Chief among these limitations is that you can monitor only a limited number of biochemicals per cell (the exact number is determined by the quantity of distinct fluorophores you have access to).
A microfluidic-based flow cytometry platform allows for a very different strategy: one can break up the target cell and examine its contents separately. The ability of microfluidics to confine and control the transport of biochemicals can in turn be combined with optical means for carrying out the analysis process.
Optical approaches to performing highly sensitive biochemical analysis on a microfluidic platform are numerous. One is represented by a high-finesse microtoroid resonator sensor that is a submillimeter-size disk. When an optical wave is launched onto the resonator’s rim, which is very smooth, the wave can cycle more than a million times around it. A molecule that attaches itself to the rim will be sampled by the light field repeatedly. Andrea Armani and her fellow researchers at the California Institute of Technology recently demonstrated the ability to detect specific, single molecules using such a device.3 This type of sensor is unique in its sensing dynamic range: it can sense a 12 orders-of-magnitude change in chemical concentration. A large number of these microtoroid resonators, each functionalized for affinity to a different biochemical, could be deployed for effective parallel sensing.
Cell manipulation and sorting
Certain flow cytometry applications could benefit greatly from the ability to sort cells after characterization for further analysis. This type of sorting requires either direct manipulation of the flow when a target cell passes by (which is slow and cumbersome), or selective application of a force that will drag the cell from its normal path into one that leads to a separate collection bin. Optical forces have proved to be a particularly elegant way of accomplishing this sorting.
We can separate optical forces into two categories. The first of these is trapping forces (optical tweezing) in which cells in a liquid environment are pulled toward the high intensity point of a focused laser beam. The second, called radiation pressure, results from momentum transfer from the incident photons to the cell.
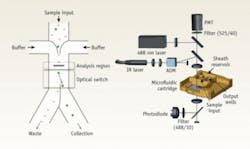
Mark M. Wang and colleagues have reported an excellent example of optical tweezing for flow-cytometer sorting (see Fig. 3).4 In this case, the identification of a desired cell upstream triggers a downstream trapping laser that deflects the cell into a secondary outlet.In a different approach, Joseph Kovac and Joel Voldman reported a system where cells were first stored in an array of microwells.5 The wells were then scanned and desired cells were removed from the wells through the use of radiation pressure (laser illumination from bottom).
An interesting extension of this approach is the idea of “optical chromatography,” which has recently been demonstrated in microfluidic formats by a number of groups.6 The technique involves flowing a mixture of cells in a microchannel that has a laser beam propagating along it in the opposite direction of the flow. When the radiation pressure force applied to the cells balances the flow force, the cells come to rest at an equilibrium position. Because the radiation pressure force applied to larger cells is greater than that applied to smaller ones, they tend to collect at different lengths along the channel based on their size. Interest in this technique is based on the realization that, for small particles, optical forces are much more sensitive to size than the separation techniques used in biology and chemistry (for example, centrifugation and electrophoresis). The limitation, however, is that with traditional optics and radiation pressure, these forces can be applied only over a very short distance (the depth of focus) and therefore the achievable resolution is relatively small.
Cost, space, and the ultimate goal
Whether we like it or not, cost consideration is a key factor in all health-care matters. One of the primary reasons that flow cytometry has proved so successful in biomedicine is that it is a low-cost analysis method compared to the far more expensive proposition of having the analysis manually performed by a pathologist.
The largely untapped potential of circulating-tumor-cell analysis is an excellent example of how economic reality can impose a cap on the quality of health care. As our community continues to bring innovations into flow cytometry, cost should always be a consideration.
Size reduction has always been touted as the key enabling factor toward the construction of compact point-of-care analysis units. However, we think that the progress of flow cytometers should go beyond that goal. We think development of implantable flow-cytometer units is a reasonable objective. An “always-on” window into the dynamics of our blood composition could much more efficiently determine and help diagnose the onset of illness before symptoms present themselves.
In the case of tumor-cell screening and analysis, implantable flow cytometers can actually go a step further and serve as a means for isolating tumor cells from the bloodstream. While it’s not quite a cure for cancer, it can be a treatment-and-containment option worth exploring.
REFERENCES
1. D. Psaltis, R.S. Quake, and C. Yang, Nature 442, p, 381 (2006).
2. Cui, X. et al., PNAS 105, p. 10670 (2008).
3. A.M. Armani et al., Science 317, p. 5839 (2007).
4. M.M. Wang et al., Nature Biotechnology 23(1) p. 83 (2005).
5. J.R. Kovac and J. Voldman, Analytical Chemistry 79(24) p. 9321 (2007).
6. B.S. Schmidt et al., Optics Express 15(22) p. 14322 (2007).
CHANGHUEI YANG is an assistant professor with the departments of bioengineering and electrical engineering at the California Institute of Technology in Pasadena; DAVID ERICKSON is director of the Integrated Micro- and Nanofluidic Systems Lab and assistant professor in the Sibley School of Mechanical and Aerospace Engineering at Cornell University in Ithaca, N.Y.; DEMETRI PSALTIS is dean of the School of Engineering, Ecole Polytechnique Federale de Lausanne in Switzerland. Contact Prof. Yang at [email protected].