Beyond cell cytometry: Tissue cytometry
A new approach enables researchers to study cells directly within thick tissues and even entire organs— and thus retain vital contextual information.
By Timothy Ragan
Cytometry, a technique used in biology and medicine to study large cell populations on a cell-by-cell basis, has proved enormously valuable in clinical and research settings. State-of-the-art cytometers from manufactures such as BD Biosciences (San Jose, CA) and Beckman Coulter (Fullerton, CA) are able to quantitatively measure thousands of cells per second and identify subpopulations as small as 1 in 100 million.
As powerful as cytometry is, however, instrument limitations generally restrict its use to cells outside their native 3-D tissue environment, which limits the range of diseases or processes that can be studied. TissueVision, the company I cofounded with Karsten Bahlmann, is developing cytometric tools that allow researchers to study cells directly within thick tissues, up to entire organs. We refer to this as tissue cytometry and it can investigate many biological and medical problems that were difficult to address with traditional cytometry.
The motivation for tissue cytometry lies in the significant drawbacks involved in removing a cell from its host tissue. Once a cell is no longer within its native 3-D tissue, its morphology is dramatically altered and all inputs from cell-matrix and cell-cell interactions are no longer present. Numerous studies have shown it is impossible to reproduce the native behavior of important cell types such as hepatic cells in the liver or neuronal cells in the brain once these inputs from the host 3-D tissue are lost. Also, the enzymatic and mechanical preparation methods used to extract cells from a tissue can change the gene expression pattern and phenotype of a cell. Moreover, beyond introducing irrevocable cellular changes, vital contextual information from the surrounding 3-D tissue and organ architecture is no longer present.
These changes can not only can confound interpretation, but for several major diseases, including cancer and heart disease, the surrounding tissue extracellular matrix is a major part of the etiology. For example, metastasis involves the recruitment of the vasculature by the cancer cells both to grow the tumor and spread to distal regions from the primary tumor. And cell pathogenesis in hepatotoxicity and cardiac ischemia is strongly mediated by fibrosis in the surrounding tissue stroma. Cells cultured on a 2-D coverslip or in suspension can never capture these types of interactions.
Modality and image quality
The appropriate choice of imaging modality is critical for obtaining high-quality 3-D images of the opaque environment of tissues. To image deep within tissues we use two-photon microscopy (TPM), a fluorescence technique invented by Winfred Denk and coworkers at Cornell University. While similar to confocal microscopy, TPM has far better depth penetration and lower phototoxicity because of the longer-wavelength femtosecond lasers used. It is not uncommon to image with 3-D subcellular detail 100 to 500 µm into a tissue. Several manufacturers, such as Carl Zeiss (Jena, Germany) and Olympus (Center Valley, PA) now offer commercial multiphoton systems, and companies such as Newport Spectra-Physics (Mountain View, CA) offer small robust turnkey Ti:sapphire lasers that provide outstanding performance and require little on the part of the user.
Despite the excellent depth penetration of TPM, it is insufficient to image entire organs that can span more than a centimeter in depth. To overcome this limitation and image tissue in all three dimensions with subcellular resolution, we use a robotics stage to move the sample from the microscope objective to an integrated microtome, and remove the topmost layer of the previously imaged tissue portion. By repeating this process and leaving a small overlap region between successive layers, we can image through entire organs. We have automated this process so that no manual intervention is required.
A major challenge we had to address was the need for high-speed imaging. Cytometry involves the analysis of large populations of cells; therefore, it was necessary to develop high-speed methods if these cells were to be imaged within a reasonable amount of time. Our solution was to split the light from a Spectra-Physics Ti:sapphire laser to generate multiple excitation spots, and to then scan these spots across the sample with a galvanometer scanner from Cambridge Technology (Lexington, MA). Traditionally, high-speed TPM or confocal imaging has required the use of specialized scanners such as polygonal or resonant scanners. Working with Cambridge Technology, however, we were able to use its 6215H galvanometer system to scan large areas of tissue very precisely and quickly.
Typically, galvanometers have involved tradeoffs between imaging speed and positioning accuracy over the range of the motion, which in turn is due to the fundamental balance that must be struck between the size of the galvanometric device and motor stiffness. With the 6215H, Cambridge was able to extend the boundaries between imaging speed and accuracy that constrained previous galvanometers, and deliver a device that allowed us to get much of the performance we previously needed a polygonal scanner for, while still affording the convenience, low cost, flexibility, and robustness of galvanometric scanning. For instance, we can scan a millimeter of tissue with positional accuracy sufficient to align successive scan lines with submicron accuracy. Unlike with polygonal scanners we can also easily change the size of the scan region and pixel residence time.
Results in 3-D
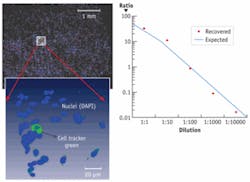
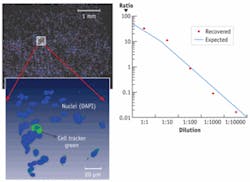
In an example of traditional cytometric analysis within a 3-D environment, we stained a population of cells with the nuclear stain DAPI and subpopulations with Invitrogen’s Cell Tracker Green in ratios ranging from 1:1 to 1:105, and seeded these populations within a 3-D collagen matrix (see Fig. 1). The samples were then imaged and automated image segmentation was used to count the number of labeled cells. We were able to recover the correct ratios and locate and visualize the rare labeled cell within the larger 3-D matrix and had a 3-D throughput rate of approximately several hundred cells/second, depending on the cell density within the matrix.
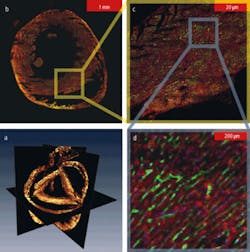
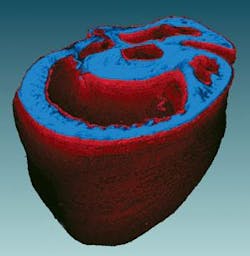
The system can acquire whole organ datasets (see Fig. 2). An entire mouse heart that spanned over 5 mm in diameter and 6 mm in depth was imaged with micron-level resolution. The dataset was almost five orders of magnitude in length scale and allowed us to simultaneously resolve individual nuclei and the smallest capillary structure, through the mesoscale structure of the cleavage planes in the walls the heart muscle, and the macroscopic structures of the heart chambers (see Fig. 3). We can map cell-level morphology across the entire heart and relate it to the local vasculature, extracellular matrix, and organ ultrastructure. This capability allows us to have entirely novel classification axes not found in traditional cytometry.
The time required to image an entire mouse heart was reduced from the six to eight weeks normally required to less than a day, and can be reduced to a few hours with further improvements and optimizations. The process is automated, and because of the straightforward nature of our approach, is compatible with virtually all tissue types and different embedding media, and does not require any special preparation or clearing techniques.
Beyond innovations in microscopy, advances in other areas have also played a role. As mentioned, the current generation of Ti:sapphire systems are closed-box, small-footprint systems with high output powers of up to 5 W of modelocked power and long diode lifetimes of more than 30,000 hours. Computational and storage requirements are no longer a limiting factor, with the availability of affordable multicore workstations and distributed computing resources, and raw storage approaching $100 per terabyte. Finally, of major importance are the advances in tissue labeling, both in ex vivo labeling kits offered from companies such as Invitrogen (Carlsbad, California) and in the rapid development of transgenic animal models for particular diseases or genotypes. These advances have increased the value and need for automated phenotyping and tissue imaging technologies that have the throughput, molecular specificity, and quantification power of fluorescence cytometry.
A sure beneficiary
One particular area that can benefit from high-throughput tissue techniques is the pharmaceutical industry. During the 1970s and 1980s drug development was more focused on in vivo and organism-level studies, which are necessarily low throughput and difficult to automate. In an attempt to increase productivity, there was a turn during the 1990s to recombinant in vitro screening systems, which, along with the combinatorial chemistry and the instrumentation at the time, could be automated and made high throughput, unlike the tissue- and organism-level studies previously conducted. “High-content” or cell-based imaging cytometry assays have become a popular and useful approach for biomarker and target validation.
A downside to in vitro studies can be lower physiological relevance: what occurs in the test tube or with cells in 2-D culture often does not translate to what happens at the organism level. As a consequence of this and other factors, the progress of drug development slowed rather than accelerated in the late 1990s and 2000s. Now, however, it is becoming possible to automate tissue- or whole-organism-level studies with approaches that TissueVision and others are developing. In the end, the benefit will be high-throughput quantitative assays with potentially greater physiological relevance to human disease.
Our work has been an exciting effort to extend the usefulness of cytometry into the complex 3-D world of tissues. Much work is yet to be done in sample handling, tissue labeling, and computational analyses of 3-D cytometric datasets, but the payoff is the quantifiability and throughput of cytometry combined with the insight and context provided by tissues.
Timothy Ragan, cofounder of TissueVision, One Kendall Square, PMB 141, Cambridge, MA 02139, has worked on advanced optical microscopy instrumentation and software analyses for more than 10 years. Contact him at [email protected].