Laser ablation technology for destroying breast tumors gets CE Mark approval
Novian Health (Chicago, IL) has obtained CE Mark approval for its Novilase Breast Therapy, a minimally invasive laser ablation procedure that uses laser-induced heat for focal destruction of breast tumors up to 2 cm in size. The approval allows the company to commercialize its technology in the European Union (EU) and Switzerland, giving women an alternative to surgery for early-stage breast cancer and benign breast tumors (fibroadenomas).
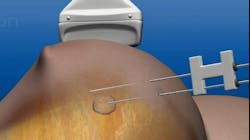
The minimally invasive laser ablation technology requires only local anesthesia. Two small probes are inserted into the breast and guided to the tumor by ultrasound imaging—one probe uses heat to destroy the tumor, while the other monitors the procedure through temperature. The treatment takes less than 30 minutes and its success is confirmed by follow-up imaging.
A recent study conducted in the U.S. and UK showed that more than 90% of malignant breast tumors were completely destroyed during one Novilase procedure. In addition, patients in the trial reported better health-related quality of life outcomes compared to lumpectomy surgery. No serious adverse events were reported.
Related: Novian Health begins laser therapy clinical trial for breast cancer
In the U.S., the laser ablation technology has been cleared as an alternative to surgery for the treatment of fibroadenomas. The FDA approved an investigational device exemption (IDE) to conduct a confirmatory study (BR-003) to gather data to support a marketing application for focal destruction of malignant breast tumors. Investigators at U.S. and European sites will participate in the BR-003 study.
In addition to obtaining the CE Mark approval, the company also received ISO:13485:2016 certification for its quality management system. To earn the certification, the company had to demonstrate its ability to provide its products and related services in a way that consistently meets customer and applicable regulatory requirements.
For more information, please visit novianhealth.com.