Clinical trial performs first procedure using Medtronic MRI-guided laser ablation to treat epilepsy
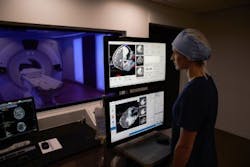
The first procedure using the Visualase MRI-guided laser ablation system from Medtronic (Dublin, Ireland) has been performed in the Stereotactic Laser Ablation for Temporal Lobe Epilepsy (SLATE) clinical trial at Mayo Clinic (Rochester, MN). The SLATE clinical trial supports expanded indication of the currently marketed Visualase system for treatment of patients with drug-resistant mesial temporal lobe epilepsy (MTLE), the most common form of partial or localization-related epilepsy.
Related: FDA approves clinical study to evaluate MRI-guided laser ablation for epilepsy
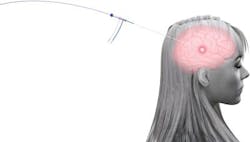
The Visualase system is cleared by the FDA to necrotize or coagulate soft tissue in neurosurgery and other surgical specialties. Through a minimally invasive procedure, laser energy is delivered to the target area using a laser applicator. As light is delivered through the laser applicator, temperatures in the target area begin to rise, destroying the unwanted soft tissue under real-time MRI guidance.
During the clinical trial, approximately 120 adult patients with drug-resistant MTLE will be treated at selected epilepsy centers across the U.S. Emory University (Atlanta, GA), Mayo Clinic, Northwell Health (Great Neck, NY), Thomas Jefferson University Hospital (Philadelphia, PA), and University of Miami Health System (Miami, FL) are some of the institutions that will be enrolling patients in the clinical trial. After the Visualase procedure, subjects will be followed for 12 months and evaluated for freedom from seizures, quality of life, adverse events, and neuropsychological outcomes.
Visualase is not cleared or approved for the treatment of epilepsy. Use of the system for the treatment of epilepsy is investigational use-only in the U.S.
For more information, please visit www.medtronic.com and https://clinicaltrials.gov/ct2/show/NCT02844465.