Femtosecond laser ablation sheds new insight on cell division
Researchers at the Harvard School of Engineering and Applied Sciences (SEAS; Cambridge, MA), using a femtosecond laser, sliced through and made quantitative measurements of the mitotic spindle (an apparatus that forms during cell division), discovering how its microtubules (protein strands) are organized in the spindles of animal cells.
The researchers first sliced through the strands of the organelle and then performed a mathematical analysis to infer the microscopic structure of the spindle from its response to the slicing.
The spindle forms during cell division and segregates chromosomes into the daughter cells. It was previously unclear how microtubules are organized in the spindles of animal cells, and it was often assumed that the microtubules stretch along the length of the entire structure, pole to pole.
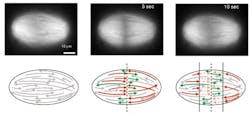
Mazur and his colleagues demonstrated that the microtubules can begin to form throughout the spindle. They also vary in length, with the shortest ones close to the poles.
"We wondered whether this size difference might result from a gradient of microtubule stabilization across the spindle, but it actually results from transport," says lead author Jan Brugués, a postdoctoral fellow at SEAS. "The microtubules generally nucleate and grow from the center of the spindle, from which point they are transported towards the poles. They disassemble over the course of their lifespan, resulting in long, young microtubules close to the midline and older, short microtubules closer to the poles."
Mazur and Brugués worked with principal investigator Daniel Needleman, Assistant Professor of Applied Physics and Molecular and Cellular Biology at Harvard, and Valeria Nuzzo, a former postdoctoral fellow in Mazur's lab at SEAS, to bring the tools of applied physics to bear on a biological question.
The team used a femtosecond laser to make two small slices perpendicular to the plane of growth of the spindle apparatus in egg extracts of the frog species Xenopus laevis. The laser allowed the team to make precise cuts and perform experiments that were not possible using previous techniques, explains Eric Mazur, Balkanski Professor of Physics and Applied Physics at Harvard, who co-authored the study.
Then, the team was able to collect quantitative data on the reconstruction of the spindle following the laser disruption and precisely determine the length and polarity of individual microtubules. Observing the speed and extent of depolymerization (unraveling) of the spindle, the team worked backwards to compile a complete picture of the beginning and end points of each microtubule. Finally, additional experiments and a numerical model confirmed the role of transport.
With further inquiries into spindle architecture, the researchers hope that scientists will one day have a complete understanding, and possibly even control over, the formation of the spindle.
"Understanding the spindle means understanding cell division," notes Brugués. "With a better understanding of how the spindle is supposed to operate, we have more hope of tackling the range of conditionsâfrom cancer to birth defectsâthat result from disruptions to the cell cycle or from improper chromosomal segregation."
The full findings appear in the journal Cell; for more information, please visit http://www.cell.com/abstract/S0092-8674%2812%2900410-2.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Follow OptoIQ on your iPhone; download the free app here.
Subscribe now to BioOptics World magazine; it's free!