KLOX BioPhotonic platform for dermatology set for expanded commercialization
Dermatology company LEO Pharma (Ballerup, Denmark) and KLOX Technologies (KLOX; Laval, QC, Canada) have entered into a worldwide license and joint venture agreement, excluding Canada, to further develop and commercialize KLOX's BioPhotonic technology platform in dermatology, which includes a CE Mark-approved, noninvasive treatment for moderate to severe acne. LEO Pharma will also make an equity investment in KLOX.
Related: KLOX Technologies launches two studies for chronic wound healing using biophotonic technology
The deal paves the way for LEO Pharma's first medical device therapy and first global market entry in acne, the most common skin disease affecting more than 150 million people worldwide. In turn, KLOX has gained global access for its acne treatment, as it builds on commercial partnerships to fuel its growth and bring its proprietary and innovative treatments to market in dermatology.
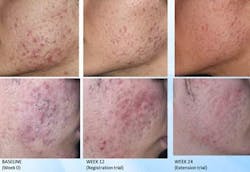
The nonabrasive, nonthermal, CE-approved acne treatment comprises a multi-LED light used in conjunction with a photoactivable converter gel. It works by targeting the underlying problems that lead to acne vulgaris, as well as stimulating collagen synthesis and healing in traumatized skin, thereby promoting healing in the epidermis and deeper in the dermis noninvasively. Completed within a 15-minute treatment cycle including preparation, treatment requires twice-weekly application over six weeks; in clinical trials, the acne BioPhotonic system demonstrated statistically significant improvements in moderate to severe acne sufferers.
The global agreement, which includes all countries except Canada, also establishes the foundation for future collaboration between LEO Pharma and KLOX in developing new dermatological treatment solutions based on KLOX's proprietary BioPhotonic platform.
Under the terms of the agreement, LEO Pharma will be responsible for clinical and commercial activities, including manufacturing. In addition, the company will provide financial funding support for KLOX research and product development in dermatology. The financial terms include a significant upfront, as well as a double-digit, escalating, tiered royalty rate based on product sales.
Dr. Lise Hébert, KLOX's president and CEO, notes that the deal will also allow the company to focus human and financial resources on the development of its wound care program, which is slated for commercialization in the first half of 2015 in Europe.
For more information, please visit www.kloxtechnologies.com/en/dermatology/acne-vulgaris.
-----
Don't miss Strategies in Biophotonics, a conference and exhibition dedicated to development and commercialization of bio-optics and biophotonics technologies!
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!