FDA clears low-level laser therapy device for nail fungus
The FDA has approved Erchonia (McKinney, TX)'s Lunula laser device, which provides low-level laser therapy to treat nail fungus.
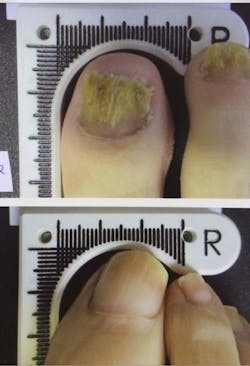
The device increases the amount of clear nail in patients infected with onychomycosis (nail fungus)—in the clinical trial for the device, 67% of patients met the success criteria of 3 mm of clear nail growth. By six months after the initial treatment, the patients averaged more than 5 mm of new growth.
Previously, treatment for nail fungus included prescription oral antifungal medication, which increased potential for liver toxicity issues, or else called for ineffective, over-the-counter topical creams.
The portable device applies a laser to the infected area. During the study, patients between 18 and 70 years old received treatments once a week for four weeks. Prior to FDA approval, the device went through four clinical trials, which recorded no known side effects.
For more information, please visit www.erchonia.com.