DISEASE DIAGNOSTICS: Nanosphere tests for success with optics, nanogold
Nanosphere’s (Northbrook, IL) Verigene system is a workstation for nucleic acid (DNA or RNA) and protein diagnostics. It is designed to allow the simultaneous detection of multiple genetic or protein targets with a single test. Comprising two core instruments—the Verigene Reader and the Verigene Processor—and a single-use test cartridge, the system is based on patented gold nanoparticle technology, which is part of a clever optics setup.
Both the Verigene System and its first test–which assesses an individual’s ability to metabolize the blood-thinning medication warfarin (sold under the brand name Coumadin) received FDA clearance in September 2007. (Warfarin is the most-prescribed oral anticoagulant in North America and Europe, and the second most common cause of emergency room visits for adverse drug events.) Since then, Nanosphere has had other tests approved, and the company went public (NSDQ: NSPH) in November 2007.
The company’s recipe for success involves a number of factors, including turnaround speed, test sensitivity, and equipment cost: Nanosphere’s relatively inexpensive and easy-to use technology could enable hospitals to conduct their own tissue-sample tests on site, instead of sending them off to labs for processing by highly trained personnel on expensive machines—and waiting for return.
How it works
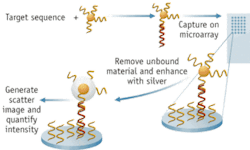
Here’s how the Verigene System works: a medical technician uses a pipette to insert a small amount of sample into the cartridge and then loads the cartridge into the Verigene Processor for analysis. After completion of the analysis mode, the slide-based microarray contained in the cartridge is transferred to the Verigene Reader, which images the slide and analyzes the results. The entire system is under the control of the Verigene Reader, which uses barcode technology to correlate patient data, test type, and the processing of the cartridge.
According to Bill Cork, vice president of R&D and the company’s CTO, the entire optics system, all reagents, and the gold nanoparticles, are contained within the disposable cartridge. In the simplest terms, what happens within the cartridge is this: the assay captures a target analyte on a glass substrate and then the gold nanoparticle complex binds with the target. The analyte is a nucleic acid sequence in the case of a genetic test or infectious disease test, and an antibody in the case of the protein test.
Nanosphere uses glass for its substrate because the material can act as a waveguide for light. “We actually use the slide as a sort of fiber-optic cable,” he explains. Light is provided by LEDs, through a waveguide which shines through the edge of the slide, he says. “Imaging controls make sure we’ve got even illumination across the plate,” says Cork. Depending on the critical entry angle of the light, a certain amount will “leak out” of the glass surface and form an evanescent wave. “This is the source of illumination.”
The gold nanospheres captured in the assay are approximately 13 nm in diameter and don’t scatter much light individually, so the company developed a process to coat the nanospheres with silver and thus amplify the light. “Typically, silver won’t bind to gold,” Cork says, “but with our process the silver will autonucleate to the gold. So we end up with particles that are upward of one-half to one micron in size, and highly reflective.” Light coming off the waveguide scatters off the silver-coated particle and creates “a one-micron sphere of light that we can detect optically. We use a simple CCD camera and we see bright stars where gold nanoparticles are.”
“Our arrays are about 10 × 25 spots,” which are typically 100 to 150 µm in diameter. Target capture analytes collect in each of those spots which, when hit with the evanescent wave, “look like a bright white spot in a field of darkness.”
System performance
The optical setup is an important part of the system’s performance. “In the very early days when the technology was first being developed at Northwestern University, we used flatbed scanners and you’d see gray spots—reflected light only. With the evanescent wave we get much brighter illumination so the CCD camera will pick up all the different spots.”
Nanosphere’s use of nanotechnology is another important contributor to system performance: Verigene is “at least 100 times more sensitive” than other techniques, says Cork. And reportedly the technology is also 100,000 times more selective, with far fewer false positives and false negatives.
Nanosphere also applies this technology to detection of proteins, which are often biomarkers of disease. “With greater sensitivity we should be able to detect disease much earlier, enabling more effective treatment and better patient outcomes,” explains Bill Moffitt, Nanosphere’s CEO. “For example, early clinical data for our cardiac troponin I assay, currently in development, indicates that it can detect cardiovascular disease within the first two hours a patient is in the emergency room,” says Moffitt.
“About 13.2 million people a year go to emergency rooms complaining of chest pain and shortness of breath, classic symptoms of acute coronary syndrome,” says Moffitt. “Only about 2% of those are actually suffering a STEMI coronary, a kind of acute myocardial infarction that can be detected on an EKG. The remaining 98% are either experiencing other forms of acute coronary syndromes such as milder forms of myocardial infarct or unstable angina or they are having non-heart-related problems such as anxiety, hiatal hernia, or gastroesophageal reflux. In order to preserve heart muscle and improve patient outcomes, early detection is very important.”
“Lacking analytical sensitivity, today’s commercially available assays often take 8 to 12 hours or more to detect NSTEMI coronaries (a somewhat less severe form of a myocardial infarct), delaying treatment decisions. Moreover, today’s assays are not sufficiently sensitive to diagnose unstable angina and many of these patients will be discharged only to experience a myocardial infarct within a few months—and many of these folks will die,” Moffitt says. “Therefore, it is no surprise that myocardial infarct is the number one driver for malpractice lawsuits against emergency physicians–and that doctors err on the side of caution.”
Nanosphere aims to quickly determine those patients suffering from acute coronary syndromes and provide accurate diagnosis of those patients suffering unstable angina. At the same time, Nanosphere believes its test will provide a very early rule-out for cardiovascular disease, thereby reducing the cost of patient care and helping to relieve congestion in emergency rooms. The company is targeting commercial launch of the product in mid-2009.
Other tests, improved ease of use
Meantime, the company has an assay for cystic fibrosis in clinical trials. A flu test is due out in early 2009, a test to detect human genetic mutations that cause hemochromatosis is in the works, and the company has scheduled for development another protein test for earlier detection of recurrent prostate cancer based upon the biomarker prostate specific antigen (PSA), which increases in concentration as the prostrate becomes diseased. “There are lots of these in market,” says Moffitt, “but once the prostrate is removed surgically, virtually all patients’ PSA drops to levels that are undetectable by standard technology—and yet 40% of such patients get recurrent cancer.” Early tests on Nanosphere’s technology indicate an ability to catch these cases within the first 90 days, he says.
The company is also exploring tests for cancer, autoimmune disease, and Alzheimer’s disease.
In the midst of all this, the company is working to improve its instrument platform. “The first generation Verigene System requires some sample prep,” says Moffitt. Verigene II will not, and thus will further simplify testing.
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.