CELL BIOLOGY/PATHOLOGY: Optical profiling enables large-scale measurement of cellular nanomechanics
An advance in optical interferometric profiling enables better understanding of nanomechanical response in cells. The high-throughput approach is promising for drug development, and for pathology prediction at the cellular level.
By Jason Reed
The nanoscale analysis of cell mechanics provides an exciting emerging method for analyzing a variety of biological and biochemical responses in cells. A team of researchers at the California Nanosystems Institute (CNSI) at University of California Los Angeles (UCLA) has been using an interference microscope to make nanomechanical measurements to evaluate the cellular effects of diseases and various drugs, and even to differentiate between cancerous and non-cancerous cells. We have developed a customized system that provides unique benefits in understanding and predicting pathology at a cellular level.
The need for innovation
Traditional techniques for quantifying cell biophysical properties include atomic force microscopy (AFM), bio-microrheology (BMR), magnetic twisting rheometry, microplate stretching, optical tweezers, optical stretching, and micropipette aspiration. These approaches have provided valuable insights into the structure and function of the intracellular matrix, cytoskeleton, and cell membrane. They have also shown the potential for characterizing and classifying diseased cell states: Study results suggest the potential existence of a detectable, quantifiable mechanical signature for cell types and diseased states.1-3 For instance, a number of studies have shown that metastatic cancer cells are more compliant than regular cells, and some researchers are looking into using this phenomenon for diagnostic purposes. We have recently discovered a number of similar responses that indicate resistance to therapy in cancer cells.
But despite enabling such gains, these techniques have a number of limitations, including the inability to measure extremely soft materials and rheological changes. They also tend to destroy samples, suffer from slow speed, and often exhibit weak linking between the measured parameter and cell mechanics.
To resolve some of these issues, we have worked with engineers at Veeco (Santa Barbara, CA), a provider of metrology and process equipment, to develop a relatively high-throughput cell analysis system able to measure. Able to process thousands of cells per day per instrument, the instrument suits the requirements of pharmaceutical screening in live cell systems. The system employs Veeco’s Wyko-brand interference microscope for non-destructive, near real-time measurements of cell nanomechanical responses to force application or drugs, without the need for staining or other types of labeling. For the observation of cells in a fluid by the interferometric optical profiler, we constructed a special reference cell for Veeco’s through-transmissive media objective that matched exactly the dispersion of the liquid cell media. Finally, a live cell perfusion chamber was built with a fixed viewing window for observation.
Building a better optical profiler
Optical profilers, which are based on specialized microscopes with interferometric objectives, use light from a single source that is split, directed, and reflected from a test object. And, they use a high-quality reference surface to generate fringes from the interference of these two beams. A camera detects the fringes created as the objective is scanned along the optical axis, and analyzes them to determine the shape of the object of interest.
Such profilers have been widely used for more than two decades by makers of semiconductors, microelectromechanical systems (MEMS), and data storage devices. The evaluation of living cells, however, presents technical challenges for standard interferometric profilers. For this purpose, a system must be able to measure objects in fluid, where the refractive index of the cell and fluid is sometimes very similar. Also, the time-scales of biomechanical properties for changing environmental conditions can vary greatly, requiring a variety of measurement methodologies to maximize data, including strobed interferometry and video-rate deformation calculations. In addition, the responses of individual cells can differ significantly from one another in a particular environment, making measurements of large numbers of cells in parallel an important requirement to achieve proper statistical sampling of a population. These realities present challenges both in how to optimize the field of view for lateral resolution and for how each surface image is analyzed, as the software intelligently tracks and logs information from each of many different cells simultaneously.
The optical profiler we use for live cell studies is, in principle, an optical microscope with a 20X 0.28NA Michelson interference objective. It allows for the recording of not only lateral features with typical optical resolution (1.16 microns for the 20X objective), but also height dimensions of reflective objects below the scale of one nanometer. Live cells are maintained in an environmentally controlled chamber with a glass observation window. The Michelson interferometer is composed of a beam splitter, reference mirror, and compensating fluid cell to adjust for optical path differences induced by fluid in the observation chamber. During each measurement, the objective head is scanned vertically from the reflective surface below the cells to a height of 40 microns above the surface, such that each point in the volume passes through focus. The interferometer is aligned so that the interference intensity distribution along the vertical scanning direction has its peak (best fringe contrast) at approximately the best focus position.
With this technology, mechanical properties of cells can be assessed in situ and in parallel using micro-indentation techniques. Samples are prepared for imaging by using microreflectors, placed above the cells as probes for measuring nanometer displacements in cell height and position. The mechanical imaging interferometry provides direct biophysical data, such as cell thickness and position, for approximately 1000 cells at any given time. Nanomechanical properties, such as the Young’s Modulus of cells, can be ascertained by imparting a magnetic field on the microreflectors (20pN – 20nN) to measure force displacement on the surface of the cells.
Put to use
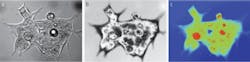
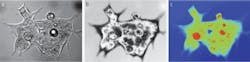
Our initial studies evaluated the efficacy of using interferometry in live cell studies.3–5 For instance, using the approach to investigate human hct116 colon carcinoma cells in a culture medium gave us considerable detail of the cell membrane, including intra-cellular organelles (see Fig. 1). This measurement represents the variations in the optical path as the incident beam travels through the fluid and the almost completely transparent cell. The signature is linear with the thickness of the cell because the whole wavefront traveling through the cell is interfered with the independent reference wavefront, and thus this type of measurement is called quantitative phase imaging.
The quantitative phase image produces something very much like an x-ray picture of the cell. Subcellular structures that are of different density show up very clearly even though they are entirely transparent in a normal micrograph. This technique allows us to very precisely track the movement of material within the cell. From this we can measure growth and response of the cell to external stimuli much more rapidly, and non-invasively, than with traditional methods. We are just learning how to correlate this information with disease state. The diseased cells themselves don’t necessarily look “unhealthy,” but their response to stimuli tends to be different.
This method enabled us to discover that the nanomechanics in hundreds of cells can be monitored simultaneously at a vertical resolution of <50 nanometers. In addition, we were able to achieve high-throughput measurement of changes in the viscoelastic behavior of treated cells.
In another recent study, local redistribution of cell content was monitored as small indentations were made by highly magnetic probes on the cell surface. First, it was found that there was almost instantaneous redistribution of cell material as a result of indentation on the surface of the cell, which was undetected with intensity imaging alone. Second, changes in local compliance were observed within 200 seconds when force was applied cyclically to regions of the cell. It would be extremely difficult to measure these types of immediate viscoelastic measurements with conventional biophysical measurement techniques, particularly at such a large scale.
Toward effective disease therapy
To understand and predict pathways toward effective disease therapy, there is a need for novel, non-invasive means for establishing molecular signatures for disease and evaluating cell response to treatments. Our interferometric approach enables high-throughput, large-scale measurement of cellular nanomechanical responses, with the potential to identify and monitor specific cells within a bulk population. These features are desirable for any system that is used to distinguish between two cell types within a heterogeneous cell mixture, or even to identify cancer stem cells.
This system has potential to become a screening tool in drug development, because it can measure cell mechanical response very rapidly and non-invasively, and is fully compatible with existing techniques such as fluorescent labeling. Today there really is no other technique that can measure a broad array of cell mechanical properties sufficiently quickly. The idea of studying mechanical properties of cells the same way we study biochemical properties of cells is relatively new, so for it to be adopted widely more basic research showing its utility will have to be published. Right now, the National Cancer Institute is making a big push to enable this kind of research. Whether it should be used as a diagnostic tool directly, or to help develop diagnostic screening procedures that are then carried out in the clinic using a specialized technique, is unclear at this point.
References
- Guck, J. et al, (2005) Biophys. J 88(5), 3689-98.
- Elson, E., L. (1988) Annual Review of Biophysics and Biophysical Chemistry 17(1), 397-430.
- Reed, J. et al, (2009) Langmuir 25(1), 36-39.
- Reed, J. et al, (2008) ACS Nano 2(5), 841-46.
- Reed, J. et al, (2008) Nanotechnology 19(23), 235101.