Infrared nerve stimulation: Hearing by light
JONATHON WELLS, MARK BENDETT, DANIEL J. LEE, JONATHAN CAYCE, AUSTIN DUKE, and AGNELLA IZZO MATIC
Over the past three decades, many researchers have attempted to modulate/stimulate neural activity using a variety of lasers, with equivocal results. Recently, researchers at Vanderbilt University (Nashville, Tenn.) spurred a new field when they successfully stimulated the rat sciatic nerve using short pulses of infrared light. Their initial studies documented the advantages of infrared nerve stimulation (INS) over standard electrical stimulation methods: improved spatial selectivity, lack of a stimulation artifact in the recorded response, and noncontact stimulus delivery.
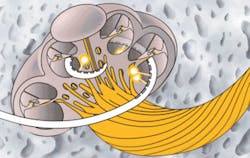
Subsequently, the emerging field of INS has inspired a partnership between industry and academia to develop its biomedical applications and the associated application-specific tools and devices. Today several institutions, including Vanderbilt, Northwestern University (Evanston, Ill.), and the Massachusetts Eye and Ear Infirmary and Harvard Medical School (Boston, Mass.), are actively investigating how INS might improve the ability to diagnose or treat patients suffering from neural dysfunction. Ultimately, the technology has the potential to offer a new set of tools and methods for wide-ranging applications such as treating pain and depression, controlling Parkinson’s tremors, improving hearing among deaf patients with new cochlear implant and auditory brainstem implant technology (see Fig. 1), and developing prosthetic limbs that provide tactile feedback to the user.
Developing the tools
Armed with initial INS successes achieved with the free-electron laser (FEL) and later with a Ho:YAG laser, Vanderbilt inventors partnered with Aculight (Bothell, Wash.), which recently became Lockheed Martin Aculight, to develop and test an application-specific, compact, low-cost optical stimulator. The effort was funded through a Small Business Innovation Research (SBIR) grant by the National Institutes of Health (NIH) and eventually became the Capella R-1850.
In designing this device, the inputs from electrophysiological testing were critical to determine all relevant stimulation parameters, including wavelength (a critical parameter mediating tissue penetration depth and efficacy of stimulation in a variety of neural cell types), pulse widths, repetition rate, and even fiber diameter (which controls fluence). Since then, several variations on the original unit have been made to adapt to the variations in the physiology of motor axons, sensory axons, and central neurons.
Brain stimulation
Building on their successful IR stimulation of peripheral motor and sensory neurons, researchers E. Duco Jansen, Anita Mahadevan-Jansen, Jonathan Cayce, and Austin Duke at Vanderbilt are developing optical techniques for stimulating and recording neurons within the brain. Numerous applications could benefit from INS in the central nervous system (CNS) mainly because of its high spatial selectivity. INS could allow more precise cortical mapping, which can be a direct benefit for patients suffering from traumatic brain injury as well as improved demarcation for brain tumor resections. One day INS may also be used for chronic stimulation as a therapeutic intervention, such as deep brain stimulation.
Recently, Vanderbilt researchers have shown optical stimulation feasibility in an in vitro preparation of thalamocortical brain slices. Action potentials locked in time with the laser pulses were observed at locations of neural projections several millimeters away from the site of stimulation. When compared to electrically stimulated responses, the optical signal included a smaller volume of stimulated neurons and lack of noise artifact in the recording. Results from this in vitro study helped pave the way to in vivo studies in the somatosensory cortex.
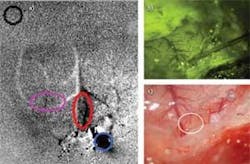
The researchers used optical imaging techniques to record and characterize the neural signals indirectly by documenting the hemodynamic response. Acquiring images under 630 nm illumination, they used a 1.94 µm pulsed diode laser for in vivo cortical stimulation in a rat (see Fig. 2). They showed that by first obtaining a hemodynamic activation map (increased blood flow correlates with increased brain activity) in response to vibrotactile stimulation, they could then place an optical fiber and deposit 10’s µJ/cm2 of energy 100 µm below the surface in the same region. The hemodynamic response to optical stimulation was then detected in the somatosensory and motor cortex. Results showed comparable time course data in response to both optical and tactile stimulation, demonstrating the ability to artificially stimulate the manifestation of touch with precision that matches the capabilities of the normal sensory feedback system (Fig. 2, bottom).Enabling precision hearing
Claus-Peter Richter and Agnella Izzo Matic at Northwestern University are exploring use of INS for a new generation of cochlear implants.
Contemporary cochlear implants use electric current to stimulate neurons. However, spread of electric current in tissue limits the number of independent stimulation contacts along the cochlea to about 10. This fundamentally limits the fidelity of sound reproduction in state-of-the-art cochlear implants. But INS, by virtue of its spatial selectivity, has the potential to overcome this limitation, opening up the possibility of independently stimulating more sites along the cochlea. Functionally, increasing the number of stimulated sites should dramatically improve speech perception and enhance the overall fidelity of hearing.
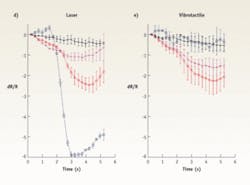
The Northwestern University investigators have demonstrated increased spatial selectivity of INS over electrical stimulation in several animal models using a variety of methods, including electrophysiologically and histologically. Recordings from inferior colliculus neurons and masking experiments demonstrate that an optically stimulated population of neurons can be confined to an area similar to that stimulated by a moderate-level acoustic tone (see Fig. 3).To adequately restore a sense of hearing and to encode sound intensity (loudness), an optical cochlear implant would need to operate up to stimulation rates of at least 200 to 400 Hz without causing damage. However, these high rates of optical stimulation raise the possibility than an increase in temperature from each laser pulse would accumulate and cause tissue damage. Continual-stimulation experiments conducted for up to six hours at up to 400 Hz indicate that INS can evoke a very stable compound action potential, which is a sensitive marker for the physiological state of the cochlea. Cochlear damage and changes in temperature would show a drastic decrease in amplitude. While these results are promising, further studies investigating the effects of chronic stimulation are needed and ongoing.
It has been shown that INS is feasible in the cochlea of acute and chronic deafened animals in which acoustic thresholds are significantly elevated. The evoked potentials from acutely deafened animals and optically stimulated chronic deafened animals were not significantly different from normal animals. This demonstrates the feasibility of optical stimulation of neural systems that have been exposed to some trauma with subsequent neural degeneration.
Better auditory brainstem implants
Daniel J. Lee, MD, a neurotologic surgeon in the Department of Otology and Laryngology, Harvard Medical School and the Massachusetts Eye and Ear Infirmary, (Boston, MA) has recently applied INS to the development of an auditory brainstem implant (ABI).
A cochlear implant requires the presence of a functional auditory nerve to conduct action potentials from the cochlea to the brainstem (cochlear nucleus). Patients who are deaf due to absent or damaged auditory nerves as a consequence of neurofibromatosis type 2 (NF2), severe cochlear dysplasia, or temporal bone fractures are not candidates for a cochlear implant. In these cases, the ABI bypasses the auditory nerve to directly stimulate the cochlear nucleus, the first relay station in the brainstem for all ascending sound information originating in the ear.
Current ABI technology uses surface or penetrating electrodes to deliver electrical current to the second-order auditory-system neurons found in the cochlear nucleus. However, functional outcomes in patients with conventional ABIs have been modest. The majority of NF2 patients with ABIs receive sound awareness that enhances communication with lip-reading, but do not have meaningful open-set word understanding. In addition, some patients experience nonauditory sensations and discomfort from the ABI due to nonspecific stimulation of neurons that are located near the cochlear nucleus. Improving the selectivity of cochlear nucleus stimulation may help improve ABI performance.
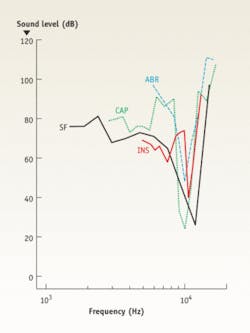
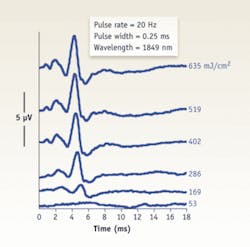
Based on these clinical observations, Lee’s team tested the feasibility of using INS to selectively stimulate regions of the cochlear nucleus in an animal model. These initial in vivo experiments used the Capella to stimulate the cochlear nucleus of an anesthestized rat. The researchers observed stable optically evoked auditory brain-stem responses (oABRs) during 30 minutes of continuous surface stimulation of the cochlear nucleus without evidence of tissue injury. The amplitude of the oABRs, depending on the location of stimulation in the cochlear nucleus, increased with increasing pulse energy levels (see Fig. 4). This work is one of the first descriptions of optical stimulation of the CNS in vivo and the data suggest that mid-wavelength infrared lasers are capable of acutely stimulating neurons of the cochlear nucleus without tissue damage. These results provide the basis for novel stimulation paradigms of the central auditory system that in the future could overcome fundamental obstacles associated with electrically based stimulation technologies.The future of INS
As more researchers enter the INS field and new devices are developed, the number of applications for this technique will likely continue to grow. One of the next steps is to establish INS safety and efficacy to move the technology into clinical trials. Following will be development and use of diagnostic tools to evaluate cranial and peripheral nerve function.
INS could allow scientists and clinicians to truly interface with the nervous system at a microscopic level they have never been able to consider without significant disruption of the microenvironment of the cellular structure. Applications that are now open for possible clinical opportunity include mapping cortical dominance columns, light-based cochlear and vestibular system stimulators, deep brain stimulator implants for treating Parkinson’s disease, or even cortical implants related to brain-machine interface applications.
Members of the team who also contributed to this article are E. Duco Jansen (professor of biomedical engineering and neurological surgery), Anita Mahadevan-Jansen (associate professor of biomedical engineering and neurological surgery), Peter Konrad (associate professor in the Department of Neurosurgery and Biomedical Engineering), and Chris Kao (research associate professor of neurological surgery) of Vanderbilt University; and Claus-Peter Richter (associate professor of otolaryngology) of Northwestern University.
References
- M. Kennedy, “The Return of the Optical Transport Market: Video, IP Services Drive New Growth Initiatives,” Telecommunications Online (Feb. 27, 2007).
- C. Medford, “Fiber Diet for Telecom,” Red Herring (Aug. 14, 2007).
Jonathon Wells and Mark Bendett are members of the Medical Products Group at Lockheed Martin Aculight, 22212 20th Ave. SE, Bothell, WA 98021. www.aculight.com. Contact them at [email protected], [email protected]. Daniel J. Lee MD is an assistant professor in the Department of Otology and Laryngology, Harvard Medical School and a neurotologic surgeon in the Department of Otolaryngology, Massachusetts Eye and Ear Infirmary; e-mail: [email protected]. Jonathan Cayce is a third-year PhD student in the Department of Biomedical Engineering at Vanderbilt University. Austin Duke is a second-year graduate student in the Department of Biomedical Engineering at Vanderbilt University. Agnella Izzo Matic is a post-doctoral fellow in the Department of Otolaryngology at Northwestern University; e-mail: [email protected].