Low-cost fluorescence test could better diagnose hepatitis B
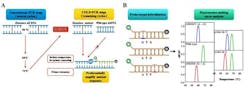
A team of scientists in China has developed a test that involves highly sensitive coamplification at lower denaturation temperature polymerase chain reaction (COLD-PCR) coupled with probe-based fluorescence melting curve analysis (FMCA) for precision diagnosis of chronic hepatitis B (CHB) patients. The simplified, low-cost test may be used routinely in hospital laboratories.
Hepatitis B virus (HBV) infection affects the liver and can be fatal, usually as a result of cirrhosis or liver cancer. The virus is typically spread through contact with infected blood or bodily fluids. HBV can be prevented by vaccines that offer almost-total protection against HBV infection.
"Guidelines have confirmed that dynamic monitoring of HBV DNA, genotypes, and reverse transcriptase (RT) mutant DNA is of great importance to assess infection status, predict disease progression, and judge treatment efficacy in HBV-infected patients," explains lead investigator Qishui Ou, Ph.D., of the Department of Laboratory Medicine, The First Affliated Hospital of Fujian Medical University, the Gene Diagnostic Laboratory, Fujian Medical University, and the Fujian Key Laboratory of Laboratory Medicine (Fuzhou, China). "We believe COLD-PCR/FMCA provides a powerful laboratory tool for precise diagnosis and treatment of HBV-infected patients."
Although a number of molecular methods have been developed for measuring these parameters, many are limited by poor sensitivity or inability to detect more than one mutation at a time. Others are too cumbersome or expensive for clinical use. "Our goal was to establish a more practical and inexpensive method with high sensitivity to detect genotype and RT mutations while detecting HBV DNA," Ou notes.
Moreover, COLD-PCR/FMCA can detect HBV mutations at much lower concentrations than other techniques such as PCR/FMCA or PCR Sanger sequencing (1% vs. 10% vs. 20%, respectively). This new technique can also distinguish different phases of HBV infection according to the proportion and type of mutations, as well as by detecting HBV DNA.
The researchers also report that the genotype and mutation detected by COLD-PCR/FMCA may predict whether a patient will respond to antiviral therapy. Analysis of serum samples from 41 patients with CHB who were receiving entecavir revealed that the drug was most effective for patients with genotype B and those with a lower percentage of RT mutations at baseline or week 4.
"Until now, there have not been high-throughput approaches to detect HBV DNA, genotype, and RT mutations simultaneously. Therefore, it is necessary to establish a more practical and inexpensive method with high sensitivity to detect genotype and RT mutations while detecting HBV DNA. COLD-PCR/FMCA has that potential," Ou says.
Full details appear in the Journal of Molecular Diagnostics.