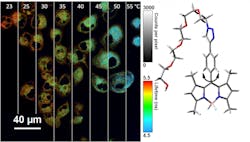
Nanothermometer uses fluorescence of molecular motors to take cell temperature
Researchers at Rice University (Houston, TX) used the light-emitting properties of particular molecules to create a fluorescent nanothermometer to take the temperature of the inside of cells. The research team, led by Angel Martí, Associate Professor of Chemistry, Bioengineering, and MSNE at Rice University, demonstrated how it modified a biocompatible molecular rotor known as boron dipyrromethene (BODIPY) to reveal temperatures inside single cells.
A molecule's fluorescence lasts only a little while inside the cell, and the duration depends heavily on changes in both temperature and the viscosity of its environment. But at high viscosity, the environment in typical cells, its fluorescence lifetime depends on temperature alone. At a specific temperature, the light turns off at a particular rate, and that can be seen using a fluorescence lifetime imaging (FLIM) microscope.
Related: Macro-FLIM provides molecular specificity with cellular resolution
Because the technique depends on the rotor, Martí and Rice graduate student and lead author Meredith Ogle constrained the rotor to go back and forth, like the flywheel in a watch, rather than letting it rotate fully. "What we measure is how long the molecule stays in the excited state, which depends on how fast it wobbles," Martí says. "If you increase the temperature, it wobbles faster, and that shortens the time it stays excited."
The effect, Martí says, is conveniently independent of the concentration of BODIPY molecules in the cell and of photobleaching, the point at which the molecule's fluorescent capabilities are destroyed. "If the environment is a bit more viscous, the molecule will rotate slower," he says. "That doesn't necessarily mean it's colder or hotter, just that the viscosity of the environment is different."
"We found out that if we constrain the rotation of this motor, then at high viscosities, the internal clock--the lifetime of this molecule--becomes completely independent of viscosity," Martí continues. "This is not particularly common for these kind of probes."
Martí says the technique might be useful for quantifying the effects of tumor ablation therapy, where heat is used to destroy cancer cells, or in simply measuring for the presence of cancers. "They have a higher metabolism than other cells, which means they're likely to generate more heat," he says. "We'd like to know if we can identify cancer cells by the heat they produce and differentiate them from normal cells."
Full details of the work appear in the Journal of Physical Chemistry B.