Fluorescence method tracks multiple in vivo interactions quickly, and at low cost
Researchers at the Rensselaer Polytechnic Institute (Troy, NY) have developed a fluorescence imaging approach that makes it possible to quickly and economically monitor multiple molecular interactions in a large area of living tissue, such as an organ or a small animal, for application in medical diagnosis, guided surgery, or preclinical drug testing. The method is capable of simultaneously tracking 16 colors of spatially linked information over an area spanning several centimeters, and can capture interactions that occur in billionths of a second.
Related: Fluorescence 'lifetime' moves toward clinical application
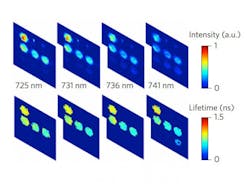
The method, developed in the lab of Xavier Intes, a professor of biomedical engineering at Rensselaer Polytechnic Institute, pairs fluorescence lifetime imaging (FLIM) and Förster resonance energy transfer (FRET) to reveal the molecular state of tissues. In FLIM, molecules of interest are tagged with fluorescent reporter molecules that, when excited by a beam of light, emit a light signal with a certain color over time that is indicative of their immediate environment. Reporter molecules can be tuned to offer information on environmental factors such as viscosity, pH, or the presence of oxygen. FLIM is ideal for the thick tissues of a body because it relies on time information rather than light intensity, which degrades significantly as it travels through tissue. FRET determines close proximity between two similarly tagged molecules, such as a drug and its target, based on an energy transfer that occurs only when the tagged molecules are delivered into the diseased cells for maximal therapeutically efficacy.
However, while the FLIM-FRET method generates a signal rich in information, collecting that signal quickly and economically is problematic. Current methods rely on expensive cameras, which can image only one reporter at a time, and scanning the subject can take hours as the camera collects information from its full field of vision.
To overcome this obstacle, the researchers dispensed with cameras and instead used a single-pixel detection method combined with a mathematical sampling technique (based on a Hadamard transform) that allowed them to collect sufficient relevant information in 10 minutes to construct a precise image. The detection method can collect information on 16 spectral channels simultaneously, and three detection devices positioned around the sample provided spatial information used to construct a three-dimensional image.
Full details of the work appear in the journal Nature Photonics.