Leica attains FDA approval for cerebrovascular fluorescence with fluorescein
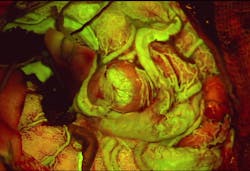
Leica Microsystems (Wetzlar, Germany) has received FDA clearance for its FL560 fluorescence microscope filter for visualization of cerebrovascular blood flow in conjunction with the dye fluorescein. When integrated into the company's M530 OH6 neurosurgical microscope, the filter provides real-time, high-contrast visualization of both cerebral anatomy in native color and fluorescent blood flow. With this combined view, the surgeon has more information to aid assessment and decision-making during vascular neurosurgery.
Related: Microscopy system harnesses virtual reality technology for image-guided neurosurgery
Assessing cerebral anatomy and vascular flow, particularly in smaller vessels and the areas they perfuse, can be challenging under white light or with traditional near-infrared fluorescence which only provides a black and white image. By integrating the filter into the previously mentioned neurosurgical microscope, a surgeon is able to view anatomical structures in white-light and fluorescent blood flow simultaneously in the oculars.
The filter can aid a surgeon's visualization and, therefore, surgical decisions in a variety of neurovascular cases, including arteriovenous malformations and aneurysms.
For more information, please visit www.leica-microsystems.com.