Device optically detects bacteria during root canal treatments
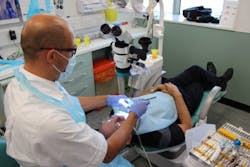
Researchers at King's College London have developed a new fluorescence-enabled device for detecting bacteria during root canal treatments, which could eradicate the need for follow-up appointments and prevent treatments from failing. The device, dubbed SafeRoot, enables rapid bacterial detection inside the root canal, ensuring the procedure has been successful and reducing the need for tooth extraction or surgical intervention.
Related: Simulations show how dental laser treatments can kill bacterial colonies effectively
Around a quarter of root canal treatments fail over time because of secondary infections, and most procedures require one or two visits to the dentist. The first appointment is used to remove infected material in the tooth and to administer an antibacterial treatment. During the second appointment, dentists visually assess the canal to check if the infection has been removed, but this process cannot guarantee that treatment has been successful. Each visit involves drilling and the removal of part of the tooth.
So, the SafeRoot device was created to detect any existing bacteria once the root canal treatment has been completed, with the aim of eliminating persistent or secondary infections and reducing the need for further treatments. Through fluorescent dyes and fluorescence microscopy and spectroscopy, the device can optically detect minute amounts of residual live bacteria in the root canal space. During clinical trials, the research team was able to detect bacterial cells after three minutes of testing.
Using conventional sterile endodontic paper points, which are routinely used in root canal treatments, the process is performed during the treatment, preventing any impact on clinical treatment time and minimizing additional clinical steps.
Frederic Festy, a senior lecturer at the Dental Institute at King's College London who led the work, says that the SafeRoot device could be applied to a wide range of biological infections as well, including wound, respiratory, and those that are implant- and contamination-related.
Full details of the work appear in the Journal of Dental Research; for more information, please visit http://dx.doi.org/10.1177/0022034517691723.