Fluorescence technique identifies misfolding proteins that cause diseases
A team of researchers from Pennsylvania State University (Penn State; State College, PA) and the University of Washington (Seattle, WA) has reengineered a fluorescent compound and developed a method to simultaneously light up two different proteins as they misfold and aggregate inside a living cell, highlighting forms that likely play a role in several neurodegenerative diseases including Alzheimer’s and Parkinson's.
Proteins fold into very precise structures to function properly, but environmental stress or pathogenic mutations can cause proteins to misfold and aggregate, explains Xin Zhang, assistant professor of chemistry and of biochemistry and molecular biology at Penn State and leader of the research team. "Protein aggregation is a multistep process, and it is believed that the intermediate form, which previous imaging techniques could not detect, is responsible for a number of diseases, including Alzheimer's, Parkinson's, Type 2 diabetes, and cystic fibrosis. We developed the Aggregation Tag method—AggTag—to see these previously undetectable intermediates—soluble oligomers—as well the final aggregates in live cells," he says.
Previous techniques to identify protein aggregation used fluorescent compounds that were always lit up, which made it impossible to distinguish properly folded proteins from the intermediate form because both trigger low-level diffuse fluorescence. The AggTag method uses "turn-on fluorescence," so the compound only lights up when misfolding starts to occur.
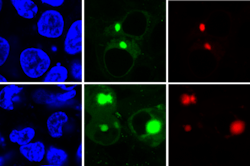
"When the fluorescent compound has plenty of space to move, it rotates freely and remains turned off, as in the presence of a properly folded protein," says Yu Liu, assistant research professor of chemistry at Penn State and the key developer of AggTag method. "But when the protein starts to misfold and aggregate, the compound's movement becomes restricted and it begins to light up. Diffuse fluorescence indicates that intermediate oligomers are present, while small points of brighter fluorescence indicate that the denser insoluble aggregates are present."
To allow for this distinction between forms, the research team reengineered the color-causing core of the green fluorescent protein (GFP), which is commonly used in imaging studies because it fluoresces when exposed to certain wavelengths of light. The reengineered compound binds to a tag, which in turn fuses to a protein targeted for imaging.
The AggTag method allows researchers to see the previously undetectable but potentially disease-causing intermediate forms of proteins as they misfold. The method uses fluorescence to simultaneously detect two different proteins (red, green) within the cell (blue). (Image credit: Zhang lab, Penn State)
The research team used two different kinds of commercially available tags, Halo-tag and SNAP-tag, which when used with AggTag can induce red or green fluorescence, respectively. Because Halo-tags and SNAP-tags do not interact with each other, they can be used to simultaneously image two different proteins with the two colors. The team also engineered the tags so that the green and red colors can be reversed, giving researchers options for future imaging.
"We plan to continue developing this method so that we can signal the transition of oligomers into insoluble aggregates using a color change," Zhang says. "This method provides a new toolbox to study protein aggregation, which is currently a highly studied topic among scientists. Hopefully this will allow us to better understand the entire process of protein aggregation and the role of each of these forms in the progression of neurodegenerative and other diseases."
Two recent papers describing the method appear in the journal ChemBioChem and the Journal of the American Chemical Society.