Laser light-activated plasmonic nanobubbles kill some cells, treat others
Seeking to find a way to kill diseased cells and treat others in the same sample at the same time, researchers at Rice University (Houston, TX) developed tunable plasmonic nanobubbles that, when activated by a laser pulse, leave neighboring healthy cells untouched. The work shows promise in replacing several difficult processes now used to treat cancer, for example, with a fast, simple, multifunctional procedure.
Plasmonic nanobubbles that are 10,000 times smaller than a human hair cause tiny explosions. The bubbles form around plasmonic gold nanoparticles that heat up when excited by an outside energy sourceâin this case, a short laser pulseâand vaporize a thin layer of liquid near the particle's surface. The vapor bubble quickly expands and collapses. Dmitri Lapotko, who led the work, and his colleagues had already found that plasmonic nanobubbles kill cancer cells by literally exploding them without damage to healthy neighbors, a process that showed much higher precision and selectivity compared with those mediated by gold nanoparticles alone, he says.
The new project takes that remarkable ability a few steps further. A series of experiments proved a single laser pulse creates large plasmonic nanobubbles around hollow gold nanoshells, and these large nanobubbles selectively destroy unwanted cells. The same laser pulse creates smaller nanobubbles around solid gold nanospheres that punch a tiny, temporary pore in the wall of a cell and create an inbound nanojet that rapidly "injects" drugs or genes into the other cells.
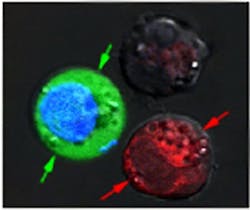
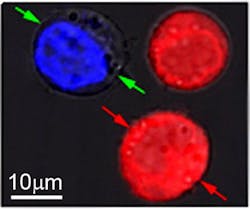
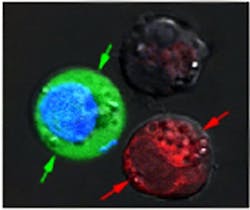
In their experiments, Lapotko and his team placed 60-nm-wide hollow nanoshells in model cancer cells and stained them red. In a separate batch, they put 60-nm-wide nanospheres into the same type of cells and stained them blue.
After suspending the cells together in a green fluorescent dye, they fired a single, wide laser pulse at the combined sample, washed the green stain out, and checked the cells under a microscope. The red cells with the hollow shells were blasted apart by large plasmonic nanobubbles. The blue cells were intact, but green-stained liquid from outside had been pulled into the cells where smaller plasmonic nanobubbles around the solid spheres temporarily pried open the walls.
Because all of this happens in a fraction of a second, as many as 10 billion cells per minute could be selectively processed in a flow-through system like that under development at Rice, said Lapotko, a faculty fellow in biochemistry and cell biology and in physics and astronomy. That has potential to advance cell and gene therapy and bone marrow transplantation, he said.
Most disease-fighting and gene therapies require ex-vivo processing of human cell grafts to eliminate unwanted cells and to genetically modify other cells to increase their therapeutic efficiency, Lapotko says. "Current cell processing is often slow, expensive, and labor-intensive and suffers from high cell losses and poor selectivity. Ideally both elimination and transfection (the introduction of materials into cells) should be highly efficient, selective, fast, and safe."
Plasmonic nanobubble technology promises "a method of doing multiple things to a cell population at the same time," says Malcolm Brenner, a professor of medicine and of pediatrics at BCM and director of BCMâs Center for Cell and Gene Therapy, who collaborates with the Rice team. "For example, if I want to put something into a stem cell to make it turn into another type of cell, and at the same time kill surrounding cells that have the potential to do harm when they go back into a patientâor into another patientâthese very tunable plasmonic nanobubbles have the potential to do that."
The long-term objective of a collaborative effort among Rice, BCM, Texas Children's Hospital and MD Anderson is to improve the outcome for patients with diseases whose treatment requires ex-vivo cell processing, Lapotko says.
Lapotko plans to build a prototype of the technology with an eye toward testing with human cells in the near future. "We'd like for this to be a universal platform for cell and gene therapy and for stem cell transplantation," he says.
The research is the focus of a paper published online by the American Chemical Society journal ACS Nano; for more information, please visit http://pubs.acs.org/doi/abs/10.1021/nn3045243.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Laser Focus World has gone mobile: Get all of the mobile-friendly options here.
Subscribe now to BioOptics World magazine; it's free!