Microscopy technique peers into membrane proteins on cell's surface
Researchers at Arizona State University (ASU; Tempe, AZ) have devised a label-free microscopy technique for examining the binding kinetics of membrane proteins on the surface of a cell. The work was led by Nongjian (NJ) Tao, Professor of Electrical Engineering and director of the Center for Bioelectronics and Biosensors at Arizona State Universityâs Biodesign Institute.
The techniqueâsurface plasmon resonance (SPR) microscopyâcould simplify the study of membrane proteins, making it promising for new drug development, aiding the identification of diagnostic biomarkers, and improving the understanding of cell-pathogen interactions.
Membrane proteins are complex structures whose subtle performance is often related to alterations in conformation and the particular binding kinetics at work. Existing techniques using fluorescent markers have been applied to pinpoint binding events, but these only permit the visualization of the protein before and after binding, omitting the dynamic processes evolving over time. Further, the use of fluorescent labels to tag protein molecules can interfere with the processes researchers hope to observe.
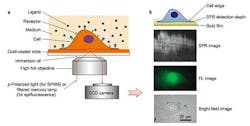
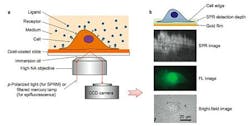
In the current study, a label-free imaging technique is applied in situ to membrane proteins, which are visualized using SPR. This effect occurs when polarized light strikes the surface of a glass slide coated with a thin metallic film of gold. Under proper conditions of wavelength, polarization and incident angle, free electrons in the metal film absorb incident photons, converting them into plasmon waves, which propagate much like waves in water.
When nanoscale phenomena, including membrane proteins, interact and disrupt plasmon waves, they cause a measurable change in light reflectivity, which the new microscopy method converts into an image.
Surface plasmon resonance had already been applied to extracted proteins to study binding kinetics, though Tao explains that many steps are required and proteins may lose their proper conformational characteristics. This is particularly true for proteins normally embedded in a cell membraneâs lipid matrix.
Another important consideration for the study of membrane proteins is the fact that that they arrange themselves heterogeneously across membrane surfaces and modify their distribution during various cellular activities. This behavior is particularly important during a process known as chemotaxis, when cells direct their movements under the influence of chemicals in the surrounding environment. For this reason, a tool allowing for both spatial and temporal study of membrane protein distribution in real time is highly desirable.
Taoâs method uses surface plasmon resonance to provide high-resolution spatial and temporal information, and also allows for simultaneous optical and fluorescence observation of the sample, combining the advantages of both label-based and label-free methods.
High spatial resolution proved particularly useful for observing the ways polarized membrane proteins (bearing hydrophobic and hydrophilic regions) rearrange themselves, assisting cell migration directed by surrounding chemicals. The phenomenon also plays an important role during immune recognition. Using SPR microscopy, the spatial distribution of membrane proteins in single cells during chemotaxis could be mapped in detail for the first time, using a chemoattractant to induce cell migration.
Cells for study are cultured directly on a gold-coated slide, which can be subjected to simultaneous brightfield, fluorescent, and SPR imaging. A liquid containing binding ligands is then applied over cells and the binding events with cell surface proteins monitored with SPR.
The technique permits millisecond resolution of temporal events and sub-micron scale analysis of spatial distribution. In the current study, the method examined the binding of membrane glycoproteins with lectin ligands, the spatial distribution of membrane receptor molecules, and membrane protein polarization and redistribution events.
The versatility of the new method, allowing for simultaneous imaging in optical, fluorescent, and SPR modes, promises to significantly expand the study of membrane proteins in their native state, improving the understanding of protein-binding kinetics and speeding the development of drugs targeting membrane proteins.
The results appear in this weekâs advanced online issue of Nature Chemistry; for more information, please visit http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.1434.html/.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Laser Focus World has gone mobile: Get all of the mobile-friendly options here.
Subscribe now to BioOptics World magazine; it's free!