Exact Sciences' noninvasive colorectal cancer screening test receives FDA approval
Exact Sciences (NASDAQ:EXAS; Madison, WI) has received FDA approval for its Cologuard stool DNA colorectal cancer screening test—the first at-home, noninvasive test for colorectal cancer that analyzes both stool DNA and blood biomarkers using fluorescence detection. Scientists at Mayo Clinic (Rochester, MN) developed the technology involved in the test, which has been licensed to the company.
Related: Next-gen sequencing helps Mayo Clinic offer personalized cancer treatment
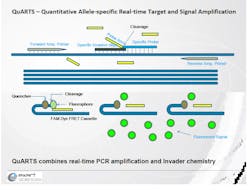
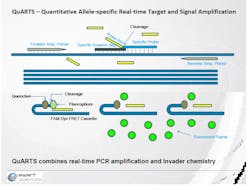
After a physician orders the Cologuard test, the kit mails directly to the patient's home. The patient then collects a stool sample in the Cologuard Collection Kit and sends the kit back to the Exact Sciences lab for testing through a prepaid mailer. At the lab, Cologuard's Quantitative Allele-specific Real-time Target and Signal Amplification (QuARTS) technology can detect biomarkers from DNA in cancer that is shed from the colon as part of the digestive process and blood released in the stool.
Multiplexed QuARTS reactions are processed using a real-time cycler, with each biomarker (NDRG4, BMP3, KRAS, and ACTB) monitored separately through independent fluorescent detection channels. The stool sample is prepared and analyzed for fecal occult blood in a quantitative Enzyme-Linked Immunosorbent Assay (ELISA) that determines the concentration of hemoglobin in the sample.
Test results are turned around in as little as two weeks, and patients learn their results directly from their prescribing physician. What's more, the test doesn't require medication, dietary restrictions, or bowel preparation prior to taking it.
The company conducted a prospective, 90-site, 10,000-patient pivotal study on the test, whose findings were published in April 2014 in the New England Journal of Medicine (http://dx.doi.org/10.1056/nejmoa1311194). "The robustly conducted research as part of this FDA approval process has proven that this noninvasive test is highly sensitive in detecting both early stage colorectal cancer and the most advanced precancerous polyps most likely to develop into cancer," says David Ahlquist, MD, a Mayo Clinic (Rochester, MN) gastroenterologist and co-inventor of the test.
The Cologuard test is available to patients through their healthcare providers in the U.S. for $599. The company has plans to make the test available in select countries in Europe pending CE Mark approval.
For more information, please visit http://www.cologuardtest.com/hcp/about-cologuard/the-science-behind-the-test.
-----
Don't miss Strategies in Biophotonics, a conference and exhibition dedicated to development and commercialization of bio-optics and biophotonics technologies!
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!