MICROSCOPY/ONCOLOGY/IN-VIVO IMAGING: Miniature microscopes for guiding brain tumor resection
Danni Wang, Steven Y. Leigh, and Jonathan T.C. Liu
Despite the availability of advanced modern medical technologies, brain tumors remain some of the most difficult cancers to treat. Survival depends on many factors, such as the tumor type and the patient's age and overall health condition. Whether the tumor is solid or diffusely infiltrative, numerous studies have shown that survival correlates with the extent of resection ("debulking surgery"), which is the first line of treatment in most cases. Unfortunately, complete resection of brain tumors is rare, as surgeons must balance the conflicting demands of removing as much cancerous tissue as possible while preserving healthy brain tissue and minimizing neurological damage.
Pre-operative MRI provides coarse image guidance during surgery, but its accuracy is limited by spatial resolution, loss of registration due to brain shifts during surgery, and poor tumor-to-normal image contrast. Histopathology is the gold standard for accurately distinguishing between tumor and normal tissues often used to examine tissue specimens at the final stages of surgery. The drawbacks of frozen-section pathology include the invasiveness of the procedure (tissue must be removed from the patient), time and expense. Histology involves numerous stages of tissue processing—including freezing, sectioning, staining, rinsing and slide mounting—and therefore can only be used to probe a few isolated points within the tumor cavity.
Surgeons and patients would benefit from a method for accurately visualizing tumor margins in real time during surgery, at numerous points throughout the tumor cavity and in a way that can guide the precise excisions necessary near eloquent regions of the brain. Ideally, this method would offer microscopic views of tissue morphology and molecular expression (structure and function) to match or exceed the accuracy of the best immunohistology techniques.
Widefield imaging of fluorescent contrast agents is being extensively studied as a means to improve tumor debulking in the brain. While promising, the inability to visualize cellular morphology and function may limit the accuracy of such techniques. To address this limitation, a number of microscopic methods are being explored for guiding brain tumor resection. We will discuss techniques based on confocal microscopy and outline other promising approaches.
Single-axis confocal microscopy
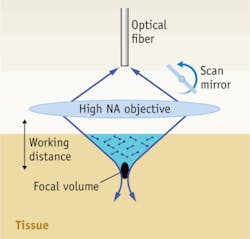
A conventional single-axis confocal (SAC) microscope can image light reflected from unstained cell and tissue structures, or from tissue structures stained with endogenously expressed fluorescent proteins or exogenously applied fluorescent reagents. Light from an illumination pinhole is focused to a sharp focal spot, while reflected or fluorescent light generated at the focus is captured by a detection pinhole (the same pinhole may be used for both). Optical sectioning is achieved by the constructive addition of two properties: 1) illumination light is strongly focused to a well-confined focal spot; and 2) a detection pinhole captures signal preferentially from the focal spot while out-of-focus and scattered light misses the pinhole and is spatially filtered away. Therefore, since both the illumination and collection pinholes are imaged to the same point in the specimen, the combined effect is a system able to image a localized focal spot deeply within a scattering media. Two- (or three-) dimensional images are reconstructed by scanning this focus through the sample, generally in a raster pattern using rotating mirrors or other mechanical scanning mechanisms. Regardless of what method of microscopy is chosen, the imaging system must be portable in order to be useful in the surgery suite, which is a formidable engineering challenge.
The conventional confocal microscope can be miniaturized using a single-mode optical fiber to serve as the illumination and detection pinhole (see Fig. 1). A miniature design has been recently demonstrated with an experimental mouse glioma model (see Fig. 2). This particular design employs a mechanical fiber scanner. Developed by Optiscan Pty Ltd (Notting Hill, Victoria, Australia), brain tissues were imaged with an axial resolution of ~7 μm, a transverse resolution of 0.7 μm, an adjustable depth of up to 250 μm and a field of view of up to 475 × 475 μm at a modest frame rate of ~1 Hz. The device's tip has a diameter of 5 mm.Dual-axis confocal microscope
The single-axis confocal architecture is not without limitations. High resolution is achieved by focusing light sharply, which necessitates the use of an objective lens with high numerical aperture (NA). These lenses are challenging to design and manufacture, especially on a miniature scale, and can be prohibitively expensive. Furthermore, the sharp focusing angle associated with high NA lenses shortens the working distance beyond the lens surface. This generally forces the scanning of the illumination and collection beams to be done before the lens, which introduces off-axis aberrations that must be mitigated by the use of multiple corrective lenses. Most importantly, the single-axis confocal microscope, while a good spatial filter, is not ideal for rejecting out-of-focus and multiply-scattered light in tissue, and is generally limited to an imaging depth of about 100 μm in biological tissues.
A dual-axis confocal (DAC) microscope design improves upon the conventional confocal architecture (see Fig. 3). The dual-axis design demonstrates several advantages over the single-axis for deep tissue imaging. First, sub-cellular resolution can be achieved in the axial and transverse dimensions with two low-NA beams. Second, simple low-NA lenses are less sensitive to aberrations and are easier to manufacture compared with their high-NA counterparts. Third, long working distances result from using low-NA beams, thus allowing the illumination and collection beams to be scanned after the focusing mechanism, which eliminates off-axis aberrations. In the case of our device, a miniature microelectromechanical systems (MEMS) mirror scans the beams rapidly over a large field of view (10 Hz frame rate). Fourth, and most importantly, the DAC architecture has been shown to be a superior spatial filter compared to the SAC architecture.4,5 Light scattered along the narrow illumination path outside of the focal volume is less likely to be collected by the narrow off-axis detection path.To further decrease the size of the tip of the microscope to make the system more amenable for surgical use, a gradient index (GRIN) needle lens has been implemented to relay the focal plane forward to the site of interest through a 2 mm diameter, needle-like optic. The biaxial-scanning MEMS mirror enables a field of view measuring 400 × 400 μm. A sliding mechanism enables adjustment of the MEMS mirror in the axial direction for changing the depth of the focal planes (0 to 250 μm). Using the DAC miniaturized microscope both ex-vivo and in-vivo, images are obtained with a transverse resolution of 4 μm and an axial resolution of 8 μm.
Alternative point-of-care approaches
Modalities other than confocal microscopy for in-vivo microscopic imaging include structured illumination microscopy and two-photon microscopy. In structured illumination optical sectioning (not to be confused with structured illumination for super-high-resolution microscopy), a patterned light source is used to illuminate the sample, where the pattern is only imaged with high contrast at the focus of the microscope and loses contrast away from the focal plane.6 By modulating the illumination pattern over time, optical sectioning is achieved because only signal originating from the focal plane will be modulated, whereas signal from out-of-focus planes will appear as a constant background. Since structured illumination is a widefield technique in which a CCD or CMOS array is used to image an entire field of view at once, the frame rate of image acquisition can be high. However, because background light is digitally filtered away, rather than actually filtered away from the detector (as is the case in confocal microscopy), the technique is limited by large amounts of background shot noise and the limited dynamic range of the detector arrays.
Two-photon microscopy has been shown to enable optical sectioning at significant depths. In traditional fluorescence imaging, a fluorophore is excited by absorbing one photon at a given energy. Two-photon fluorescence occurs when a dye simultaneously absorbs two photons whose energies add up to the same energy as in the single-photon case. Since this process is extremely weak, and scales with the square of the intensity of light, fluorescence signal is localized to the high-intensity focus of a high-peak-power pulsed laser. Since out-of-focus fluorescence signal generation is repressed, efficient optical sectioning is achieved. Furthermore, since lower-energy (near-infrared) photons are used, there is reduced tissue scattering at such wavelengths. The limitation of two-photon microscopy for clinical applications has been, and continues to be, the need for expensive and bulky pulsed laser sources and sophisticated dispersion-compensating optics.7
A simple alternative, epifluorescence microscopy—a widefield microscope in which a detector array is imaged to a sample—can be used if optical sectioning is not necessary. Rapid imaging is possible, but is limited to the tissue surface, where image contrast may be poor due to a lack of optical sectioning, and where surgically disrupted cells may not accurately display their molecular biomarkers.
Technical needs
Miniature confocal microscopy promises to provide surgeons with an invaluable means to visually access tumor margins during surgical procedures (see Fig. 4). However, the power of this technique may not be fully realized without the development of targeted contrast agents for molecular discrimination of diseased tissues. This is a large and challenging field of research that will likely play a huge role in the success of these technologies.Another technical need is for multispectral microscopes that can simultaneously visualize multiple reagents. This would enable the multiplexed imaging of a panel of biomarker targets, which may be necessary for accurate delineation of tumor margins. In addition, the multispectral approach could enable quantitative ratiometric imaging methods for increased diagnostic accuracy.8
Finally, an important future direction is to combine microscopic image detection with therapy through the use of a hybrid (theranostic) device and reagent platform. An elegant solution would be to use lasers both for optical imaging and for light-based therapy, such as photodynamic therapy or thermal lysis with metallic nanoparticles.
REFERENCES
1. T. Sankar et al., Neurosurgery 66 (2), 410-7; discussion 417-8 (2010)
2. J. T. C. Liu et al., J. Biomed. Opt. 15 (2), 026029 (2010)
3. W. Stummer et al., Lancet Oncol. 7 (5), 392-401 (2006)
4. J. T. C. Liu et al., J. Biomed. Opt. 11 (5), 054019 (2006)
5. J. T. C. Liu et al., J. Biomed. Opt. 13 (3), 034020 (2008)
6. N. Bozinovic et al., Opt. Express. 16 (11), 8016-25 (2008)
7. M. T. Myaing et al., Opt. Lett. 31 (8), 1076-8 (2006)
8. J. T. C. Liu et al., Biophysical Journal 96 (6), 2405-14 (2009)
DANNI WANG and STEVEN Y. LEIGH are both Ph.D. students, and JONATHAN T. C. LIU is Assistant Professor of Biomedical Engineering at Stony Brook University (SUNY), http://bme.sunysb.edu/. Contact Dr. Liu at [email protected].
![FIGURE 2. A hand-held miniature single axis confocal microscope used to image a glioma tumor implant in a mouse. (A) The probe is being used under an operating microscope, and the real-time optical display is visible in the background. (B) The probe tip is in contact with the exposed mouse brain, while (C) a scalpel blade cuts a thin biopsy specimen. Images taken from the same region of the glioma tumor show (D) H&E stain (scale bar 250 micrometers), (E) confocal image with acriflavine contrast agent (scale bar 100 micrometers) [1]. FIGURE 2. A hand-held miniature single axis confocal microscope used to image a glioma tumor implant in a mouse. (A) The probe is being used under an operating microscope, and the real-time optical display is visible in the background. (B) The probe tip is in contact with the exposed mouse brain, while (C) a scalpel blade cuts a thin biopsy specimen. Images taken from the same region of the glioma tumor show (D) H&E stain (scale bar 250 micrometers), (E) confocal image with acriflavine contrast agent (scale bar 100 micrometers) [1].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/pennwell.web.350.362.png?auto=format,compress&fit=max&q=45?w=250&width=250)
![FIGURE 3. (A) The dual-axis configuration uses separate low-NA beam paths to excite and collect light off-axis. The focal volume (point spread function) of the illumination beam is represented by the blue oval; the green oval shows the focal volume of the detection path. The effective focal volume of the microscope results from the intersection of these paths (black oval). Light scattered along the narrow illumination path (blue) outside of the focal volume (black oval) is less likely to be collected by the narrow off-axis detection channel (green), thus enhancing imaging depth. (B) The miniature DAC scan head uses a 1.8-mm diameter GRIN relay lens. A bi-axial MEMS mirror scans a horizontal (en face) image while a piezoelectric actuator adjusts the axial position of the MEMS mirror for depth selectivity. (C) The microscope package [2]. FIGURE 3. (A) The dual-axis configuration uses separate low-NA beam paths to excite and collect light off-axis. The focal volume (point spread function) of the illumination beam is represented by the blue oval; the green oval shows the focal volume of the detection path. The effective focal volume of the microscope results from the intersection of these paths (black oval). Light scattered along the narrow illumination path (blue) outside of the focal volume (black oval) is less likely to be collected by the narrow off-axis detection channel (green), thus enhancing imaging depth. (B) The miniature DAC scan head uses a 1.8-mm diameter GRIN relay lens. A bi-axial MEMS mirror scans a horizontal (en face) image while a piezoelectric actuator adjusts the axial position of the MEMS mirror for depth selectivity. (C) The microscope package [2].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/pennwell.web.550.151.png?auto=format,compress&fit=max&q=45?w=250&width=250)
![FIGURE 4. (a) Ptc+/- p53-/- Math1-GFP mouse spontaneously develops medulloblastoma, the most common pediatric brain tumor, with co-expression of green fluorescent protein (GFP) through the Math1 promoter gene that is over-expressed in these tumors. (b) in-vivo image of GFP-expressing medulloblastoma in a mouse at a depth of 30 μm. (c) in-vivo image of tumor vasculature in a mouse brain following intravenous administration of fluorescein-dextran; imaged at a depth of 100 μm (scale bars are 50 μm). Images acquired with a surgical DAC microscope [2]. FIGURE 4. (a) Ptc+/- p53-/- Math1-GFP mouse spontaneously develops medulloblastoma, the most common pediatric brain tumor, with co-expression of green fluorescent protein (GFP) through the Math1 promoter gene that is over-expressed in these tumors. (b) in-vivo image of GFP-expressing medulloblastoma in a mouse at a depth of 30 μm. (c) in-vivo image of tumor vasculature in a mouse brain following intravenous administration of fluorescein-dextran; imaged at a depth of 100 μm (scale bars are 50 μm). Images acquired with a surgical DAC microscope [2].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/pennwell.web.550.165.png?auto=format,compress&fit=max&q=45?w=250&width=250)