Two-photon microscopy is promising for insight into autism, schizophrenia
Researchers in the Salk Laboratory of Genetics at the Salk Institute for Biological Sciences (La Jolla, CA) found that—using two-photon microscopy—new brain cells began with a period of overgrowth, sending out a plethora of neuronal branches before the brain pruned back the connections. The observation suggests that new cells in the adult brain have more in common with those in the embryonic brain than scientists previously thought, and could have implications for understanding neurodevelopmental diseases such as autism, intellectual disabilities, and schizophrenia.
Related: High-resolution serial two-photon tomography images whole brains fast
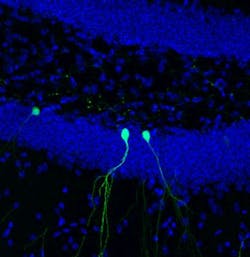
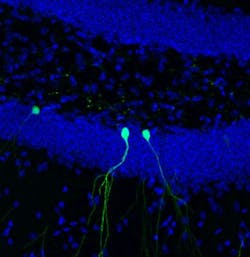
While most of the brain's billions of cells are formed before birth, senior author Rusty Gage, a professor in the Laboratory of Genetics and holder of the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease, and others previously showed that in a few select areas of the mammalian brain, stem cells develop into new neurons during adulthood. In the new study, Gage's group focused on cells in the dentate gyrus, an area deep in the brain thought to be responsible for the formation of new memories. The scientists used two-photon microscopy to observe new cells being formed in the dentate gyrus of adult mice.
"This is the first time we’ve been able to image dentate neurons growing in a living animal," says Tiago Gonçalves, a research associate in the Gage lab and first author of the new paper.
Gonçalves and Gage followed—on a daily basis—the growth of neurons over several weeks. When animals were housed in environments with lots of stimuli—running wheels, plastic tubes, and domes—the new cells grew quickly, sending out dozens of branches called dendrites, which receive electrical signals from surrounding neurons. When kept in empty housing, the new neurons grew slightly slower and sent out, on average, a few less dendrites. But, in both cases, the dendrites of the new cells began to be pruned back.
"What was really surprising was that the cells that initially grew faster and became bigger were pruned back so that, in the end, they resembled all the other cells," says Gonçalves. He and his colleagues went on to show that changing signaling pathways could mimic some of the effects of the complex environment—cells grew more initially, but also pruned back earlier.
So why would the brain spend energy developing more dendrites than needed? The researchers suspect that the more dendrites a neuron starts with, the more flexibility it has to prune back exactly the right branches. "The results suggest that there is significant biological pressure to maintain or retain the dendrite tree of these neurons," says Gage.
Defects in the dendrites of neurons have been linked to numerous brain disorders, including schizophrenia, Alzheimer's, epilepsy, and autism. Charting how the brain shapes these branches—both during embryonic development and in adulthood—may be the key to understanding mental health. "This also has big repercussions for regenerative medicine," says Gonçalves. "Could we replace cells in this area of the brain with new stem cells and would they develop in the same way? We don't know yet."
Full details of the work appear in the journal Nature Neuroscience; for more information, please visit http://dx.doi.org/10.1038/nn.4301.