Super-resolution microscopy reveals more on how the immune system is regulated
When the body is fighting an invading pathogen, white blood cells—including T cells—must respond. Now, researchers at the Salk Institute for Biological Studies (La Jolla, CA) used super-resolution microscopy to image how vital receptors on the surface of T cells bundle together when activated. Their work could help scientists better understand how to turn the immune system's activity up or down to treat autoimmune diseases, infections, and cancer.
Related: Super-resolution microscopy settles debate over nuclear pore complex, clarifies immune cell function
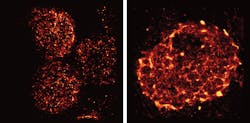
T cells are activated when receptors embedded in their outer membrane bind to other immune cells that have digested an antigen, such as a virus, bacteria, or cancer cell. In turn, the activated T cells switch on cellular pathways that help the body both actively seek out and destroy the antigen and remember it for the future. In the past, by looking at T-cell receptors embedded in isolated cells under the microscope, researchers discovered that the receptors are arranged in clusters—called protein islands—that merge when the cells are activated.
So, senior author Björn Lillemeier, an associate professor in Salk's Nomis Laboratories for Immunobiology and Microbial Pathogenesis and the Waitt Advanced Biophotonics Center, wanted more detail on how the receptors are arranged in tissue and how that arrangement might change when the T cells are activated in living hosts. The team used a super-resolution microscope developed in the laboratory of co-senior author Hu Cang, assistant professor at the Waitt Advanced Biophotonics Center and holder of the Frederick B. Rentschler Developmental Chair. The approach, called light-sheet direct stochastic optical reconstruction microscopy (dSTORM), let the researchers watch T cell receptors in the membranes of T cells in mouse lymph nodes at a resolution of approximately 50 nm.
The new images confirmed the previous observation that protein islands of T-cell receptors merge into larger "microclusters" when T cells are activated. But it also showed that, before cells are activated, the protein islands are already arranged in groups—dubbed "territories" by Lillemeier's research team.
The organization of surface receptors enables T cells to launch fast and effective immune response against antigens. Understanding how the molecular organization mediates the sensitivity of T cell responses could help researchers make the immune system more or less sensitive. In the case of autoimmune diseases, clinicians would like to turn down the immune system's activity, while turning up the activity could help fight infections or cancers.
The research could also have implications for understanding other receptors in the body, which have a wide range of functions both within and outside the immune system.
Full details of the work appear in the Proceedings of the National Academy of Sciences; for more information, please visit http://dx.doi.org/10.1073/pnas.1512331113.