New fluorescent protein could improve super-resolution microscopy of live cells
One major challenge in live-cell super-resolution microscopy is the absence of optimal fluorescent probes. Limited by the inherent optical properties of existing reversibly switchable fluorescent proteins (namely Dronpa and rsEGFP), such as a small number of switching cycles, low fluorescence signal, and poor contrast, it is difficult to achieve the desired resolution in live-cell super-resolution microscopy.
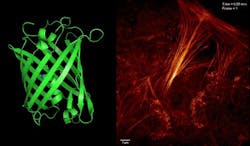
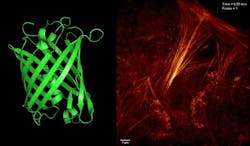
Recognizing this, a team of researchers from the Institute of Biophysics (IBP) at the Chinese Academy of Sciences (CAS; Beijing, China) and collaborators from the Howard Hughes Medical Institute (HHMI; Ashburn, VA) has developed a new type of monomer reversibly switchable fluorescent protein they call Sky Lantern for Nonlinear Structured Illumination (Skylan-NS).
In a study, they evaluated the photophysical properties of Skylan-NS against rsEGFP2 and Dronpa, both of which have been used previously in reversible saturable optical fluorescence transition (RESOLFT) microscopy and nonlinear structured illumination microscopy, respectively. With Skylan-NS, they achieved low energy (100 W/cm2), high sampling speed (sub-second level), high resolution (~60 nm), and long-term (~30 point in time) super-resolution microscopy in living cells.
The research work, which was published in the Proceedings of the National Academy of Sciences, demonstrates that Skylan-NS enables substantial improvements in the speed, duration, and noninvasiveness of live-cell super-resolution microscopy.
To view the paper, please visit http://dx.doi.org/10.1073/pnas.1611038113.