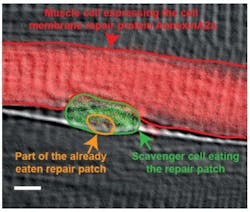
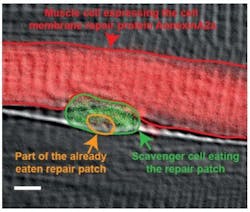
Fluorescence microscopy shows how scavenger cells repair muscle fibers
Researchers at the Karlsuhe Institute of Technology (KIT; Germany) were able to observe the muscle repair process using fluorescence microscopy, demonstrating that it only takes a few seconds until proteins from the inside of the injured cell form a repair patch that finally closes the hole in the membrane. They also showed that scavenger cells moving around within the muscle virtually perform nanosurgery to remove this repair patch later and restore the normal cell membrane structure.
Related: Light-activated neurons control paralyzed muscles
The cells of our skeletal muscles have effective mechanisms for the repair of ruptures in their cell membranes. These ruptures are because of the mechanical stress to which we expose our muscles, even when doing healthy exercises. The cell membrane is an important barrier that is essential to the proper functioning and survival of cells. If this barrier collapses and cannot be repaired quickly, the muscle cell will die, resulting in a loss of muscle mass. People whose repair proteins—for example, dysferlin—do not work properly develop atrophy of the muscles, which leads to most severe disabilities and premature death.
In an interdisciplinary cooperation project of the KIT research teams led by Uwe Strähle and Gerd Ulrich Nienhaus, PhD students Volker Middel and Lu Zhuo observed membrane repair processes with ultra-high resolution in real time in human cells and in muscle cells of zebrafish embryos. They proved that the repair patch assembling itself from repair proteins, such as dysferlin or annexines, also accumulated the phosphatidylserine lipid. Phosphatidylserine is a known appetizer for scavenger cells, the so-called macrophages.
The researchers were able to show how the macrophages indeed latch to the repair patch and eat it up. Only after the patch has been removed, the cell envelope is fully restored. Thus, the repair of the membrane in muscle fibers requires, in addition to the formation of repair patches in the injured cell, the aid of macrophages roaming around within the muscle. They further demonstrated that a short amino acid sequence in the dysferlin repair protein is responsible for the phosphatidylserine transport. It is remarkable that there are myopathy patients who have a defect precisely in this sequence of the dysferlin protein, so the new findings could contribute to the development of therapies against muscle atrophy.
Full details of the work appear in the journal Nature Communications; for more information, please visit http://dx.doi.org/10.1038/ncomms12875.