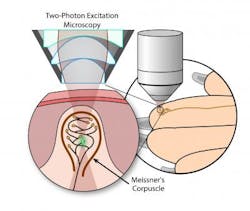
Two-photon microscopy images touch-sensitive sensory receptors in mouse fingertips
Researchers at the Nagoya Institute of Technology (NITech) and Nagoya University (NU; both in Japan) have developed an in vivo imaging method to observe Meissner's corpuscles (MCs; mechanoreceptors near the surface of the skin that are responsible for sensitivity to light touch) in living skin. Not only could the method unlock the mechanism of mechanoreceptor function, but it could also be used as a novel diagnostic tool for neural diseases and accelerate the study of aging-related neurodegeneration.
Related: New lenses improve two-photon microscopy to image larger area of neuronal activity
Previous studies, which involved observing MCs in cut sections of fixed tissues, have described the morphology and physiological functions of MCs, but the mechanism of mechanical transduction by MCs in living tissue remains unknown. The NITech-NU research team has now opened a window to understanding the mechanism of mechanoreceptor function by using two-photon microscopy (which can image living tissue up to 1 mm deep with high resolution and low phototoxicity) to observe MCs in action, in situ in the fingertips of live mice.
"To visualize MCs, we used a nontoxic and long-lived fluorescent lipophilic dye that allows for extended time-lapse observation in the same individual," says Pham Quang Trung, a PhD Student at NITech and the lead author of the paper describing the work. "The fluorescent dye persisted in injected mice for at least five weeks, and we successfully imaged the same MCs in a mouse paw three times over five days."
The basis of MC mechanotransduction is that light mechanical pressure on the skin causes physical deformation of a MC that results in an action potential. The NITech-NU scientists plan to investigate these pressure-induced changes in MC architecture with their new imaging methodology.
"We have designed and built a specialized chamber that isolates and stabilizes a mouse's paw for two-photon imaging," Pham explains. "This imaging chamber could be modified to provide controlled weight or vibration stimulations that result in different MC morphology transformations, which could then be monitored by two-photon microscopy." It could also be possible, he adds, to combine the in vivo imaging method with microneurography to describe the relationship between changes in MC architecture and the traffic of nerve impulses.
A current limitation of the in vivo imaging method is that the lipophilic dye used only labels the neural components of the MC. So Akihito Sano, the principal investigator of the study, says that creating a more complete model of MC structure and function will take effort to focus on developing two-photon-compatible in vivo labeling methods for other MC components, such as lamellar cells and the collagen capsule.
The ability to image MCs in vivo over extended periods of time, without toxicity or physical damage, has applications to human health. Human MCs decline in density with normal aging, and studies have reported changes in the shape, size, and density of MCs in a number of neural diseases, such as peripheral neuropathies.
Full details of the work appear in the journal IEEE Transactions on Haptics; for more information, please visit http://dx.doi.org/10.1109/toh.2016.2574718.