Adaptive optics enhances super-resolution microscopy for cell imaging
Building on the existing super-resolution microscopy method, an international team of researchers has developed a new ultra-high resolution "nanoscope" capable of taking 3D images of an entire cell and its cellular constituents in unprecedented detail. The advance could reveal biological phenomena never before seen and bring new medical insights.
Related: Super-resolution microscopy tracks single molecules in 3D with nanoscale accuracy
The technology—developed by researchers at Yale University (New Haven, CT), Purdue University (West Lafayette, IN), the University of Cambridge (England), the Jackson Laboratory (Farmington, CT), Howard Hughes Medical Institute (Chevy Chase, MD), and the University of Oxford (England)—incorporates several innovations in fluorescence microscopy and super-resolution microscopy. It harnesses the same kind of adaptive optics technology used in astronomy—deformable mirrors that change shape to compensate for light distortion. In astronomy, the deformable mirrors are used to compensate for atmospheric distortion to yield clear images of celestial objects. Deformable mirrors also can be used to counteract the distortion caused when light passes through biological tissue.
Super-resolution microscopy earned its developers the 2014 Nobel Prize in chemistry and has become an important tool in cell biology research. Its use, however, has been limited because of challenges in resolving features deep below the surface of samples, but the international research team has now solved that problem with the development of the new system, called whole-cell 4Pi single-molecule switching nanoscopy (W-4PiSMSN), says Fang Huang, an assistant professor of biomedical engineering at Purdue. The paper's co-lead authors are Huang and Yale postdoctoral research associate George Sirinakis. The corresponding author is Joerg Bewersdorf, a Yale associate professor of cell biology and biomedical engineering.
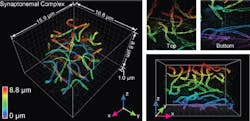
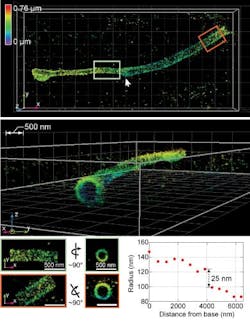
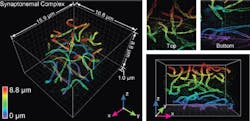
The technology was demonstrated by imaging cellular components, including synaptonemal complexes, which link chromosomes together; mitochondria and the endoplasmic reticulum, which are critical for cellular functions; the cilia, which protrude from cells like tiny antennas; and bacteriophages, which are viruses about 50 nm in diameter that infect bacteria.
The new system advances original super-resolution microscopy by incorporating the deformable mirrors into a microscope using two objectives, one above and one below the sample, and introducing a set of new algorithms to pinpoint molecular positions of proteins deep inside cells. It allows researchers to resolve details far smaller than 200 nm, representing a powerful and versatile new laboratory tool, Huang says. It allows imaging of cellular constituents in 3D at 10–20 nm resolution throughout entire mammalian cells, powerful enough to reconstruct the fine features of viruses. Resolving such fine details was once only possible using electron microscopy, which requires samples to be treated, killing the cells.
Molecules inside cells and in structures called organelles can be tagged with either photoswitchable fluorescent proteins or organic dyes that are able to glow when exposed to a small amount of ultraviolet (UV) light.
"These special fluorescent tags (fluorophores) have two states, on and off," Huang says. "And you can control the on and off states by shining light on these molecules. The concept of single molecule switching nanoscopy is to stochastically switch molecules on and off at different time frames, pinpoint the exact locations of single molecules, and reconstruct the cellular constituents at super resolution."
The super-resolved images are reconstructed from the positions of thousands to millions of single molecules.
With the W-4PiSMSN development, the research team now wants to study the cytokinetic apparatus, a core machinery during cell division, Huang says.
Full details of the work appear in the journal Cell; for more information, please visit http://dx.doi.org/10.1016/j.cell.2016.06.016.