Ultrafast laser technique enables nondestructive 3D imaging of cells
A team of researchers at Walailak University (Nakhon Si Thammarat Province, Thailand) and Hokkaido University (Sapporo, Hokkaido, Japan) has shown that an ultrafast laser technique can achieve micron resolution of single cells, imaging their interiors in slices separated by 150 nm, unlike the typical 0.5 mm spatial resolution of a standard medical magnetic resonance imaging (MRI) scan. The work may open the door to new ways of studying the physical properties of living cells with in vivo imaging.
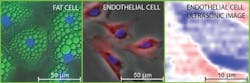
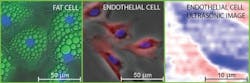
Their work centers on imaging two types of mammalian biological tissue—a bovine aortic endothelial cell, a type of cell that lines a cow's main artery blood vessel, and a mouse "adipose" fat cell. Endothelial cells were chosen because they play a key role in the physiology of blood cells and are useful in the study of biomechanics. Fat cells, on the other hand, were studied to provide an interesting comparison with varying cell geometries and contents.
The team accomplished the imaging by first placing a cell in solution on a titanium-coated sapphire substrate and then scanning a point source of high-frequency sound generated by using a beam of focused ultrashort laser pulses over the titanium film. This was followed by focusing another beam of laser pulses on the same point to pick up tiny changes in optical reflectance caused by the sound traveling through the cell tissue.
"By scanning both beams together, we're able to build up an acoustic image of the cell that represents one slice of it," explains co-author Professor Oliver B. Wright, who teaches in the Division of Applied Physics, Faculty of Engineering at Hokkaido University. "We can view a selected slice of the cell at a given depth by changing the timing between the two beams of laser pulses."
The team's work is particularly noteworthy because "in spite of much work imaging cells with more conventional acoustic microscopes, the time required for 3D imaging probably remains too long to be practical," Wright says. "Building up a 3D acoustic image, in principle, allows you to see the 3D relative positions of cell organelles without killing the cell. In our experiments in vitro, while we haven't yet resolved the cell contents—possibly because cell nuclei weren't contained within the slices we viewed—it should be possible in the future with various improvements to the technique."
So far, the team has used infrared (IR) light to generate sound waves within the cell, "limiting the lateral spatial resolution to about one micron," Wright explains. "By using an ultraviolet-pulsed laser, we could improve the lateral resolution by about a factor of three—and greatly improve the image quality. And switching to a diamond substrate instead of sapphire would allow better heat conduction away from the probed area, which, in turn, would enable us to increase the laser power and image quality."
So lowering the laser power or using substrates with higher thermal conductivity may soon open the door to in vivo imaging, which would be invaluable for investigating the mechanical properties of cell organelles within both vegetal and animal cells.
Next, the research team hopes to try their method on single-celled organisms or bacteria.
Full details of the work appear in the journal Applied Physics Letters; for more information, please visit http://dx.doi.org/10.1063/1.4918275.
-----
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn