Fluorescence imaging method can visualize motor neuron motion in real time
Researchers at the Salk Insitute (La Jolla, CA) have developed a fluorescence imaging method to watch the activity of motor neurons (those that control muscles) in real time. The method is helping them understand how spinal cord cells make connections with motor neurons, and how clinicians might be able to repair those connections in patients with spinal cord injuries or neurodegenerative diseases like amyotrophic lateral sclerosis (ALS).
Related: Light-activated neurons control paralyzed muscles
In the past, to measure the activity of neurons—whether in the brain or extending throughout the body—scientists relied on electrodes that could detect the change in electrical voltage inside a cell when it's activated. But it is tricky to use electrodes to simultaneously record the activity of many different neuron types at once to study how their activity is synchronized.
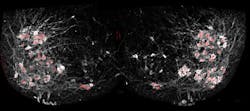
To get around this shortcoming of electrode readings, Samuel Pfaff, a professor in Salk's Gene Expression Laboratory, and his team used a fluorescent sensor protein called GCaMP6f that lights up whenever a neuron is activated. Unlike the electrodes, the protein could easily be added to many different cells at once. When Pfaff and his colleagues added GCaMP6f to motor neurons, they could watch with a microscope which cells were activated in a mouse spinal cord when chemicals that turn on walking circuits were added. What's more, Pfaff says, the method does not require any post-image processing for interpretation, as the signals are raw and able to been seen through a microscope.
Pfaff's group used the new method to answer a long-standing question about how a collection of cells in the spinal cord, called the locomotor central pattern generator (CPG), connects to the right motor neurons to allow movements like walking. The CPG, Pfaff says, is where relatively simple signals from the brain—to walk forward, or move your hand off a hot stove—are translated into more complex instructions for motor neurons to control muscles.
"Our nervous system has to make decisions and computations to tell different muscles to contract, or when not to contract, or the amount of force and speed to use when contracting," Pfaff explains. It's the CPG that helps make many of these computations, scientists believe. So normal movement requires that CPG neurons in the spinal cord connect to and control when motor neurons fire. But, until now, researchers didn't know exactly how the CPG cells forged these connections.
By tweaking the locations and identities of motor neurons, and then watching the resulting patterns of activation using their new fluorescence technique, Chris Hinckley in the Pfaff laboratory found that the CPG didn't rely solely on the cells' locations to connect to them. Instead, the genetic identity of each subtype of cells—what makes those that control the quadriceps muscle different from those that control the calf muscle, for instance—is also important.
That's a key finding, Pfaff says, for research on how to treat spinal cord injuries and ALS. Currently, many scientists are attempting to turn stem cells into motor neurons, which they then implant into the spinal cord to regenerate damaged connections. Pfaff's new results, though, suggest that general motor neurons might not do the trick—the best treatment may require the right subtypes of motor neurons. More work, however, is needed to understand the implications of this and exactly how it might translate to disease treatment.
Full details of the work appear in the journal Neuron; for more information, please visit http://dx.doi.org/10.1016/j.neuron.2015.08.005.
Follow us on Twitter, 'like' us on Facebook, connect with us on Google+, and join our group on LinkedIn