DISEASE DIAGNOSIS: Better fluorescing, brighter nanophotonics improve diagnostics
Recent advances in nanobiophotonics, including metal enhanced fluorescence and high-brightness nanoparticles, are enabling significant improvement in the analytical performance of inexpensive fluorescence biochips.
By Brian D MacCraith and Colette McDonagh
Fluorescence techniques play a key role in biomedical diagnostics. But while fluorescence-based detection is generally more sensitive than alternative optical approaches, the analytical performance of point-of-care (POC) optical biochip platforms is often lacking. Such systems feature biosensors with patterned arrays of immobilized bio-recognition elements (e.g. antibodies, aptamers, or single-stranded DNA) that bind respective targets in a patient sample–and they generate fluorescence by way of corresponding fluorescent labels. Two factors are often to blame for lackluster performance: the low levels of fluorescence generated from the thin layer of labeled complexes on the biochip surface, and low concentrations of biomarkers in the sample.
Recent advances in nanobiophotonics, however, are enabling significant enhancements in the analytical performance of fluorescence biochip platforms, particularly with regard to the sensitivity and the limit of detection (LOD). In this article, we'll describe two key enhancement approaches. First is metal enhanced fluorescence (MEF), whereby metal nanostructures or nanoparticles are used to enhance the fluorescence via localized surface plasmon resonance effects. Second, we'll look at the replacement of conventional single molecule dye labels with high-brightness dye-doped silica nanoparticles, in order to yield improved bioassay performance.
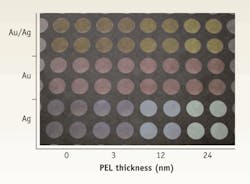
FIGURE 1. An upturned microplate coated with three different types of nanoparticles (gold/silver alloy in the top two rows, pure gold in the two middle rows, and pure silver in the bottom two rows) and with increasing numbers of PEL over-layers, ranging in thickness from 0 to 24 nm. (Image courtesy Langmuir, ACS).
Metal-enhanced fluorescence (MEF)
Localized surface plasmon resonance (LSPR) occurs when light of an appropriate wavelength interacts with a metal nanoparticle that is small compared to the wavelength of the light. The resulting resonance produces an increase in the extinction coefficient of the particle at the resonance wavelength (λres), which in turn is highly dependent on the nanoparticle's composition, size, and shape. If the nanoparticle is adjacent to a fluorescent molecule, the LSPR effect gives rise to an increase in electric field around the molecule, which, under certain conditions, can enhance the fluorescence of the molecule. The enhancement mechanism can involve either an increase in excitation rate which occurs when λres coincides with the absorption band of the molecule, or an increase in quantum efficiency when λres overlaps the emission band of the molecule. More commonly, a combination of both of these effects can give rise to the enhancement. The effect is highly sensitive to the separation between the nanoparticle and the fluorescent dye molecule. There is an optimum separation for maximum enhancement–which can depend on the experimental configuration.
An important criterion for achieving optimum plasmonic enhancement is to match the nanoparticle λres to either the dye absorption or the emission band. It is possible to accomplish this by varying the particle's parameters–such as shape, composition, and size. This is exemplified by the fact that clear liquid containing 15 nm particles appears yellow in color, whereas that same liquid with 53 nm particles instead appears blue. The extinction peak shifts to the red as the size of the nanoparticle increases. Furthermore, higher wavelength resonances in the red region of the spectrum are characteristic of non-spherical NPs. For example, triangular-prism-shaped NPs can be spectrally matched to commonly used red dye labels such as cyanine-5 (Cy5). Using a variety of synthetic techniques, it is possible to tune λres across the visible spectrum.
Exploiting the MEF enhancement principle for improved optical biochip performance, however, requires a reproducible technique for depositing such customized nanoparticles on the biochip surface. We accomplished this by coating plastic biochips with polyelectrolyte (PEL) layers using a layer-by-layer technique. As a proof of principle, we used transparent microwell plates initially as a convenient experimental platform for optimization purposes. As a measure of the reproducibility achieved, it is worth noting that the standard deviation of the measured absorbance, calculated over 16 wells, ranged from 1.5% for pure silver (Ag) nanoparticles, to a maximum value of 3% for pure gold (Au).The gradual color change we observed from left to right for a given nanoparticle type indicates the sensitivity of λres to the refractive index (or dielectric constant) of its environment. Overall, the experiment provided is a visible indication of the reproducibility of both the PEL layer-by-layer technique and of the subsequent nanoparticle deposition process (see FIGURE 1).
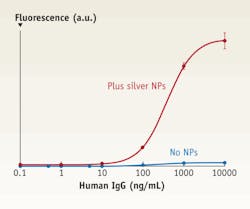
FIGURE 2. Typical dose response curves correlate to metal-enhanced assays (upper trace) and standard (that is, without nanoparticles) assays (lower trace).
Assay improvement using MEF
To demonstrate and quantify the benefits of plasmonic enhancement for improved bioassays, we used the nanoparticles and deposition system described above to devise a metal nanoparticle-enhanced fluorescence-based sandwich immunoassay based on a model polyclonal IgG-goat anti-human IgG system. The base layer of the assay platform–a layer of silver nanoparticles–was deposited as described above on a PEL layer.
We chose Cy5 for our fluorescent label, and we adjusted the LSPR of the deposited nanoparticles to match the dye's spectroscopic properties. The capture antibody binds covalently to the nanoparticle surface. For the assay without nanoparticles, the capture antibody binds to the PEL surface (see FIGURE 2).
For the highest antigen concentration relative to the same assay performed in the absence of nanoparticles, we have demonstrated a maximum fluorescence enhancement of 37. This translates to an improved LOD of 0.2 ng mL-1 compared to the value of 6 ng mL-1 achieved without nanoparticles. From our work and that of others in the field, it is clear that the combination of the MEF and a reproducible nanoparticle deposition protocol has potential to produce fluorescence-based bioassays with dramatic enhancements in analytical performance.
High-brightness nanoparticle labels
In fluorescence-based bioassays, the conventional approach is to use single-molecule fluorescent labels that are conjugated to the secondary bio-recognition elements. This approach can limit the analytical performance of the assay, however. Brighter fluorescent labels–which can provide orders of magnitude improvement in the numbers of photons emitted–yield substantial performance enhancements.
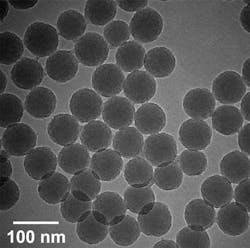
We have developed a range of dye-doped silica nanoparticle labels that contain large numbers of single dye molecules in a silica matrix (see FIGURE 3). These so-called high-brightness nanoparticles (HBNPs) have many advantages over single-molecule labels, including, for example, superior brightness, enhanced photo-stability, biocompatibility, ease of functionalization, and non-toxicity. The nanoparticles' silica surface can be functionalized easily with appropriate bioactive groups using conventional organosilane chemistry. The two principal approaches we have used to synthesize various HBNP types are the Stöber process and the water-in-oil microemulsion technique. Both are variations of the well-known sol-gel fabrication process.
FIGURE 3. The high brightness nanoparticles (HBNPs) contain large numbers of single dye molecules in a silica matrix and provide many advantages over single-molecule labels.
In particular, we have used the microemulsion method to synthesize nanoparticles doped with the near-infrared dye NIR 664 (a low-cost alternative to Cy5). Red and NIR-emitting dye labels have significant advantages for use in fluorescence-based immunoassays compared to labels that emit at shorter wavelengths, as the red emission does not overlap the intrinsic fluorescence of biological molecules, hence minimizing background interference during detection. Furthermore, whole blood has very weak absorption in the NIR, thus reducing the requirement for blood separation filters. The synthesis protocol was tailored in order to optimize dye brightness by minimizing FRET effects within the HBNPs. In HBNPs of diameter 60nm, for example, we have found brightness enhancements of the order of 160 (although much greater enhancements are achievable with dyes that have larger Stokes Shifts than NIR 664 or Cy5).
In proof-of-principle experiments, we have performed fluorescence immunoassays, using the NIR 664-doped HPNPs as a fluorescent label, in order to detect changes in the concentration of human IgG antibody, for example. We used a sandwich assay format and compared the results with those obtained using the commercial NIR organic dye label Cy5.
The nanoparticles were labeled with polyclonal goat anti human IgG antibodies, and bovine serum albumin (BSA) was used as a blocker to reduce non-specific binding on the assay substrate. Although the HBNP is ~160 times brighter than a single dye molecule, this does does not translate directly to assay LOD enhancement. Steric hindrance–owing to the relatively large diameter of the HBNP–interferes with antibody binding and compromises the brightness. Nevertheless, it is possible to achieve100-fold LOD reductions in such assays.
Future directions
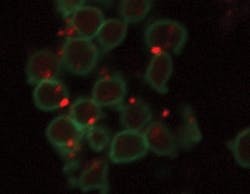
Finally, in an alternative application of HBNPs to bioassays, we are currently examining their use in novel platelet function assays. Blood platelets play a major role in the human vascular system. In particular, the presence of activated platelets in the blood may be an indicator of the onset of stroke and cardiovascular abnormalities. Platelet function assays are commonly used to detect the presence of activated platelets. We have been examining the use of HBNPs in order to enhance the detection efficiency of such assays. Current work is focusing on the conjugation of the HBNPs to antibodies for various platelet receptors and quantification of the efficiency of binding of the nanoparticle-antibody to the platelet using techniques such as flow cytometry and confocal microscopy (see FIGURE 4).
FIGURE 4. Antibody-labelled NIR dye-doped silica high-brightness nanoparticles (HBNPs) appear as orange labels, attached here to blood platelets (green).
The ultimate objective is to develop a simple whole blood assay that enables direct measurement of the degree of platelet activation in an individual, thereby facilitating a thrombotic risk characterization.
Advances in the science and technology of nanobiophotonics continue to play a key role in the development of improved techniques for biomedical analysis, especially in the areas of diagnostics and imaging. In the case of POC diagnostics, substantial improvements in the analytical performance of inexpensive biochip platforms are possible through the clever exploitation of such developments. Scale-up and reproducibility remain the major technical challenges.
Brian MacCraithis director of the Biomedical Diagnostics Institute (BDI) and the Optical Sensors Laboratory, and Colette McDonagh is deputy director of the Optical Sensors Laboratory, at Dublin City University in Ireland, www.bdi.ie and www.osl.dcu.ie; [email protected].
More Brand Name Current Issue Articles
More Brand Name Archives Issue Articles