IN-VIVO IMAGING/MOLECULAR IMAGING: Speeding the path to clinical trials: optical imaging in drug development
STEPHEN OLDFIELD
Drug development is increasingly competitive, and techniques that provide efficiency and speed are increasingly critical in bringing new compounds to market. Leading pharmaceutical companies use imaging techniques to identify at an early stage which compounds justify further investment.
As a drug approaches clinical trials, pharma companies concentrate on directly translatable approaches (i.e., those widely used in the clinic: PET, CT, and MRI). But some of the most exciting drugs approaching the clinic today—in areas as diverse as oncology, infectious disease or osteoporosis—are getting there sooner because of optical imaging techniques. Scientists at pharmaceutical companies such as Cubist, Pfizer, Novartis, Amgen, and Abbott (see “Some drugs helped by optical techniques”) report three major benefits of optical molecular imaging:
Efficiency: Optical imaging provides sufficient throughput to support animal studies early in drug development. Performing pathway analysis, compound efficacy, and pharmacodynamic studies in vivo early in development provides an efficient screen for compound optimization. Results can take half the time of traditional approaches providing rapid progress toward clinical trials with a more robust drug candidate.
Economy: Longitudinal studies using optical reporters provide more rigorous data from fewer animals than traditional methods. And a study with up to 70% fewer animals saves on compound synthesis, time, and outsourced services. Longitudinal studies provide robust statistics and results can often be seen earlier than with traditional histology.
Insight: Optical imaging can provide mechanistic insight into the mode of action to inform decision making right up to clinical trials. Co-registering optical data with traditional imaging modalities such as computed tomography (CT) provides the final translational step to the clinic.
Pre-clinical approaches
Optical molecular imaging (a.k.a. whole animal imaging) at its best uses a combination of fluorescent and bioluminescent reporters to provide sensitive, cost-effective, and quantitative monitoring of disease progression and responsiveness to therapeutic agents in living animals. Because it measures non-invasively and in real time without exposing the animal to harmful radiation, it enables longitudinal and extended studies.
Fluorescence is used to visualize cells in vivo, to analyze compound biodistribution, and to tag a variety of specific probes. Fluorescent probes can either bind to cellular features such as receptors or be modified by cellular enzymes such as proteases to give a specific signal. Fluorescence also serves as a histological marker for validation studies.
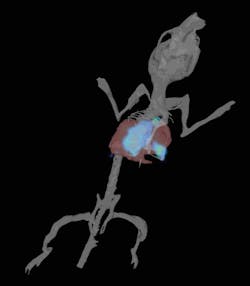
Bioluminescence is used to track metabolically active cells or monitor gene expression. Bioluminescence can be exquisitely sensitive in the detection of labeled cells—sensitive enough to enable visualization of a single sub-cutaneous cell non-invasively. Used to track the progress of infectious agents it can reveal reservoirs of residual disease and it can show tumor metastases at the earliest stage of development in lymph nodes or bones (see Fig. 1).
Equipment requirements
An ultra-sensitive camera is needed to detect the low light emissions of bioluminescence: Typically a back-thinned CCD cooled to -90°C is used to reduce the background for maximum signal to noise. Large format (6 inch diameter optics), low aperture lenses (f1 – f8), and high efficiency filters (with >90% transmission) maximize the available signal, while the animal is temperature regulated and maintained under anesthesia during the imaging process. A calibrated system is necessary to compare results over a longitudinal study, so the software must correct for aperture and exposure time to establish absolute units. Tomographic methods are used to localize the signal in three-dimensional space and provide reconstructions that can be co-registered with CT, magnetic resonance imaging (MRI), or other clinical imaging techniques. With instruments calibrated to standards verified by the National Institute of Standards and Technology (NIST), necessary for absolute quantitative measurements, optical data are comparable across experiments and from location to location.
Biological tissues are typically autofluorescent, so for quantitative fluorescence measurement, spectral unmixing is used to separate signal from background. Because longer wavelength light penetrates tissue more efficiently, red or near-IR reporters are preferred to the green fluorescent protein commonly used in microscopy.
Transillumination of the animal provides better sensitivity for imaging at depth, and 3D tomography provides the kind of absolute quantitation that can report picomoles of dye or number of cells in a precise location.
While 3D analysis enables co-registration, 2D analysis provides the rapid results necessary for high throughput studies with multiple cohorts of animals.
Because optical molecular imaging offers high throughput at low cost (for instance no dedicated operator and the ability to image five mice in as many minutes), it can be used across all areas of the drug development pipeline. Levels of throughput that would be both time constrained and cost prohibitive with techniques like positron-emission tomography (PET) or single photon-emission computed tomography (SPECT) become routine in pharmaceutical labs using optical methods.
Optical reporters at work
In the following examples, optical reporters might signal quantitative gene expression, cell number, anatomical co-registration, and 3D localization with multiple reporters employed in the same animal.
Longitudinal studies: Repeatedly imaging the same animals in a longitudinal study provides greater statistical significance from relatively few animals and generates a more biologically relevant understanding of a therapy.
For example, an infectious disease study that would otherwise require multiple steps of animal sacrifice, organ harvesting, and bacterial plating can be done with 70% fewer animals. The results are visible in real time and in addition, the experimental animals are available for studies of residual disease and remission/relapse that might be extended for months.
Tumor models: Monitoring tumor growth by optical molecular imaging provides exceptional insight because of the weeks taken to monitor disease progression. Orthotopic tumor models can be deep within the animal and optical imaging provides the ability to monitor gene expression and ask biochemical questions about disease progression. In models of disperse disease such as metastatic tumors, sensitive bioluminescence imaging provides precise location and quantitation of metastases that would be hard to locate by traditional means.
Pathway analysis: In drug development it is important to explore and understand the drug's mechanism of action. Non-invasive real time analysis of underlying signaling pathways is another application for optical imaging, which can provide a direct readout from the animal. Optical reporters can be multiplexed to allow for multiple pathway readouts in the same study.
Pharmaceutical companies commonly use markers like p53, MAPK, HIF-1, or NFkb linked to luciferase as reporters in primary screens. By injecting cells embedded in matrigel plugs into animal models companies have a simple pharmacodynamic tool to evaluate compounds early in optimization.
Molecular targeting: Additional reporters that provide further context to a study could include targeted antibodies or peptides with fluorescent or bioluminescent labels. Peptides have been constructed to selectively bind to bone, metalloproteases, arterial plaque, or a site of inflammation. Spectral unmixing of the optical reporters allows removal of background autofluoresence and the resolution of five or more reporters in a single animal.
More efficient clinical trials: Co-registration with clinical modalities becomes important as a compound progresses toward the clinic. When optical imaging is used to study the effect of a drug or drug combination, it can be used to evaluate dosing or treatment regimes to support more informed decision making in the clinical trials.
For the future
Because optical molecular imaging can't image all the way through a patient as it can through a mouse, it will never by itself meet every need in the clinic–though it still has some attractive applications. For instance a number of companies are exploring optical scanners for breast imaging, and another opportunity is for intra-operative imaging where optical techniques might be used to delineate the margins of a tumor during surgery. These methods are limited only by the scarcity of optical probes or reagents approved for use in the clinic.
Meanwhile whole animal imaging using optical reporters is gaining acceptance in all therapeutic areas as new reporters and imaging probes add to the repertoire of assays. The speed and economy of the technique ensure that it moves upstream in the development process, allowing pharmaceutical researchers to monitor their compounds in animals sooner and make faster progress toward clinical trials. And instruments now under development will combine imaging modalities to provide more quantitative information or to unravel more complex biological problems.
REFERENCES
- Mortin et al., Antimicrobial Agents and Chemotherapy, May 2007, 1787–1794.
- Mendel et al., Clin. Cancer Research, Vol. 9, 327–337 (2003).
- Xin et al., Clin Cancer Res (12) August 15, 2006, p. 4908–4915.
- Palma et al., Clin Cancer Res 2009; 15(23) December 1, 2009.
- Miller et al., Mol Cancer Ther (7),. July 2008.
Stephen Oldfield, Ph.D., is Senior Director, Imaging Marketing, at Caliper Life Sciences, www.caliperls.com, [email protected].